
July 27, 2016 — Paul Sundberg and Bob Morrison
The Morrison Swine Health Monitoring Project (MSHMP) was developed in 2012 to monitor the incidence of PRRS virus. It has grown in representation across the country and today includes 29 systems with approximately 1,000 sow herds and 2.6 million sows. With primary support from the Swine Health Information Center, one of its objectives is to help monitor the emergence and movement of swine diseases across the U.S. Beginning late summer last year, the MSHMP started tracking the cases of Senecavirus A (Senecavirus A, SVA) through reporting from the project’s participants and the Iowa State University, University of Minnesota and South Dakota State University swine veterinary diagnostic labs.
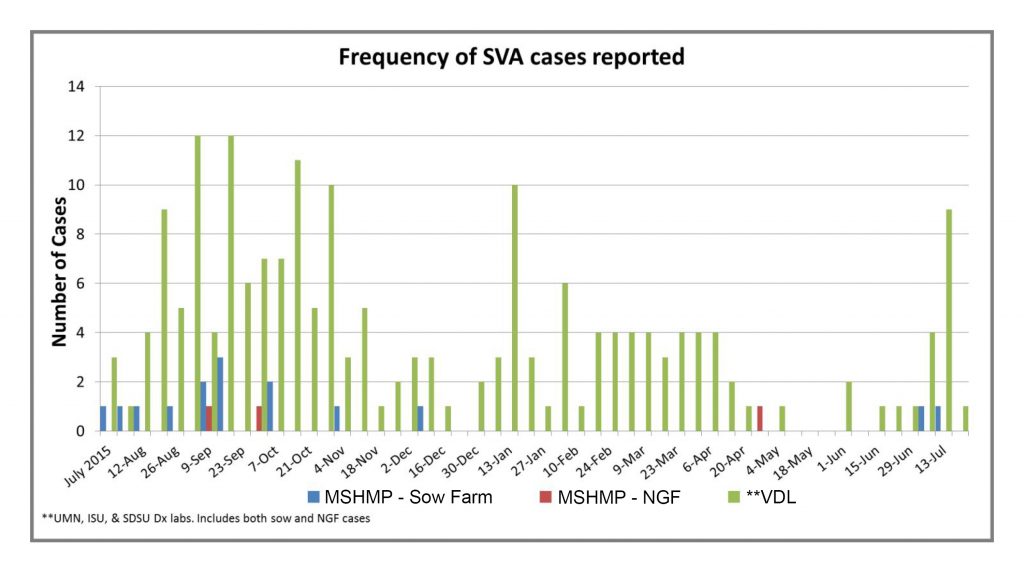
These data include reports of positive accessions into the three participating veterinary diagnostic labs. They don’t indicate if a positive accession is a new case or a previously identified case that is continuing to monitor. However, there is concern that, after a continued but relatively low incidence during the winter months, the spike in late July might be a predictor of increased incidence as we experienced last year.
Follow Proper Protocols, Know the Plan Cases of pigs or sows with vesicles are now showing up at packing plants. U.S. packing plants must continue to be able to operate normally with the number of pigs and sows being processed every day. Vesicular lesions in pigs or sows ready for marketing should be investigated, and if possible, the lesions should be allowed to resolve before shipping. If that isn’t possible, communication between the packing plant, SAHO and USDA is essential if pigs or sows are to be shipped without significant disruption to the normal operations of both the plant and the producer involved.
The USDA has issued a guidance document, Recommendations for Swine with Potential Vesicular Disease for its field staff that provides a good description of their plan to properly handle potential FAD investigations where information suggests that SVA may be suspected. This guidance tells those doing the investigations that duplicate samples may be taken when appropriate, sending one to a National Animal Health Laboratory Network Veterinary Diagnostic Lab (NAHLN VDL) and another to the Foreign Animal Disease Diagnostic Laboratory on Plum Island, New York. The federal and state animal health officials may use the clinical presentation and the NAHLN diagnostic test result to make initial decisions about movement of the pigs. The USDA lab on Plum Island, however, is the official lab that can confirm a negative FMD test.
A full series of webinars reporting on SVA research funded by the Swine Health Information Center and other sources (including length of shedding, effective disinfection, epi investigations and a case study of herd closure to eliminate the virus) and the latest on USDA guidance for investigation and responses are available on the SHIC website, https://www.swinehealth.org. Also on the website are fact sheets on SVA and other Swine Disease Matrix viruses.
The mission of the Swine Health Information Center is to protect and enhance the health of the United States swine herd through coordinated global disease monitoring, targeted research investments that minimize the impact of future disease threats, and analysis of swine health data. For more information, visit https://www.swinehealth.org or contact Dr. Sundberg at [email protected].
Copyright 2024 | Swinehealth.org | Website by Heartland Marketing Group