
Senecavirus A (SVA) is a small, non-enveloped picornavirus, unknown until 2002 when it was discovered incidentally as a cell culture contaminant. Only a single species, Senecavirus A, is currently classified in the Senecavirus genus of the family Picornaviridae, although sporadic serologically similar isolates have been identified in porcine samples spanning almost three decades. Naturally occurring antibodies against the virus have been detected in swine, cattle, mice, and a single human sample, though the virus is not known to cause disease in humans. Pathogenicity in swine remains unclear. Outbreaks of idiopathic vesicular disease have been linked to SVA in the absence of other identified etiologic agents and also during concurrent infection with porcine circovirus and porcine enterovirus. In contrast, the virus has also been identified in healthy pigs, and experimental infection has failed to produce clinical signs thus far.
Swine SVA infection has occurred across the United States and Canada, and idiopathic vesicular disease has been reported globally from Europe to South America to Australia and New Zealand. Transmission of picornaviruses is generally very rapid and occurs in the cytoplasm of host cells. Clinical signs of SVA, when present, are indistinguishable from those of swine vesicular disease (SVD), vesicular stomatitis virus (VSV), vesicular exanthema of swine virus (VESV), and foot-and-mouth disease virus (FMDV), all more serious and economically devastating foreign animal diseases (FADs). Erosions, ulcerations, and vesicular lesions of the snout, oral mucosa, and distal limbs, especially around the coronary band, may be observed. Hoof sloughing and lameness can also occur, as well as more general symptoms of illness such as fever, lethargy, and anorexia.
Cultivation and purification of SVA can be performed in the laboratory using human retinoblast (PER.C6® ) cells and human lung cancer cell monolayers (NCI-H1299a ), yielding high virus titers. Replication of SVA occurs readily in certain human tumor cells with neuroendocrine properties that are most sensitive to killing by the virus, while leaving normal adult human cells relatively unscathed. Electron microscopy, immunohistochemistry (IHC), reverse transcription polymerase chain reaction (RT-PCR), and quantitative real-time RT-PCR have been used in the study and diagnosis of SVA. Monoclonal antibodies have been developed in an attempt to develop more rapid and sensitive immunoassays for diagnosis, leading to the creation of a successful competitive enzyme-linked immunosorbent assay (cELISA) for specific detection of anti-SVA antibodies.
Understanding the epidemiology of SVA and potential role of other species in virus transmission and origin, combined with continued development of rapid and specific diagnostics and elucidation of the link between viral infection and clinical disease in swine, will be crucial to our knowledge and ability to manage this newly discovered and little understood virus.
Click for more complete information
Please download this PDF for some important information about the current incidence of SVA (Senecavirus A). For perspective, keep in mind that in 2015 we’ve had confirmed SVA from only 20 – 30 cases from across the U.S. The incidence is low but it is much higher than the 2 or 3 sporadic cases per year that is our historical experience. The Swine Health Information Center is funding research and epi investigations to help us understand more about this virus and be better prepared and informed if we continue to see new cases.
Better preparedness with research and information is important but only one part of what we need. NPPC, NPB, AASV and SHIC have been working to draft plans for proposed industry responses to new or emerging diseases. One of the components of that response plan is a panel of producers and veterinarians to discuss and recommend to state animal health officials and USDA appropriate responses based on the information available about a new or emerging disease. In short, whether a new or emerging disease is local, regional or national and whether we have the knowledge and tools to rapidly and effectively respond. Unfortunately, SVA has shown up before that body could be formed.
In its absence, the AASV Swine Health Committee was asked if they would help inform responses by reviewing the available information and decide the scope and distribution of SVA already in the U.S. They also were asked to make recommendations about responses. That is what is attached.
There are two take home messages. First, they have defined the SVA incidence this summer as a “Type 3” status – national in scope with limited knowledge and tools. The details of a Type 3 status are in the AASV statement. That’s important to help put into context discussions about the effectiveness of local, specific responses like holding pigs from movement while waiting for them to stop shedding the virus.
The second is their repeated recommendation that pigs with illness or active SVA lesions should not be marketed until the lesions at least start to resolve. We can’t afford to have a packing plant shut down because of SVA being mistaken for FMD and stopping processing and commerce. At the same time, we can’t also become complacent and assume that active lesions are SVA, thus not alerting state or federal officials, whether on the farm or in the packing plant. Doing so puts pork producers, veterinarians and all of our industry at risk. Discussions are taking place with USDA and the packing companies about appropriate communications and responses about pigs that have had SVA, are healing, have had a FMD-negative test and are going to be presented to the market.
This isn’t “news”. It’s as it always has been. But there is heightened risk of complacency if we continue to find SVA after checking for FMD and that risk is unacceptable and avoidable.
This is an opportunity to underscore the message of careful observation and immediate reporting of clinical signs or out of ordinary events. The National Pork Board has multiple resources, including barn posters and other written and visual materials. They’d welcome sending you these materials to help with your effort.
On June 8, 2017, 10 loads of finishing pigs were tested by PCR for (SVA) at a packing plant in the Midwest United States. Six of the 10 were positive. Subsequently, a total of 74 lots from 61 suppliers tested positive for SVA between June 8, 2017, and July 10, 2017. This prompted an investigation to describe the outbreak and to identify factors that may have contributed to the spread of the virus during the outbreak. The investigation term was broken down to three time periods, the PreOutbreak Period from April 24 to June 7 (45 days), the Outbreak Period when positive cases were reported from June 8 to July 10 (32 days), and the PostOutbreak Period, after the last positive case was reported, from July 11 to August 8 (35 days).
This document offers a starting point to address the investigation of foreign animal diseases—SVA specifically—in a modern era where investigatory methods have not changed to meet the needs of commerce in a fast‐paced and efficient pork industry. What used to be unusual (FAD investigations) has become routine, resulting in a greater drain on limited resources (staffing, laboratory, financial). The greatest challenge exists where the need to protect the industry from catastrophic disease events intersects with commerce.
Systematic epidemiological investigation of cases of Senecavirus A in US swine breeding herds.
What to expect when blisters are reported to state
and federal officials.
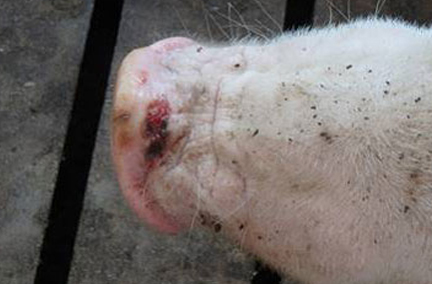
The incidence of Senecavirus A (Senecavirus A) has increased in 2015 compared to historical incidence in other years. SHIC is working with academics, researchers, USDA and others to help understand the scope of the cases and the role SVA has in causing clinical disease.
Copyright 2024 | Swinehealth.org | Website by Heartland Marketing Group