
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments.
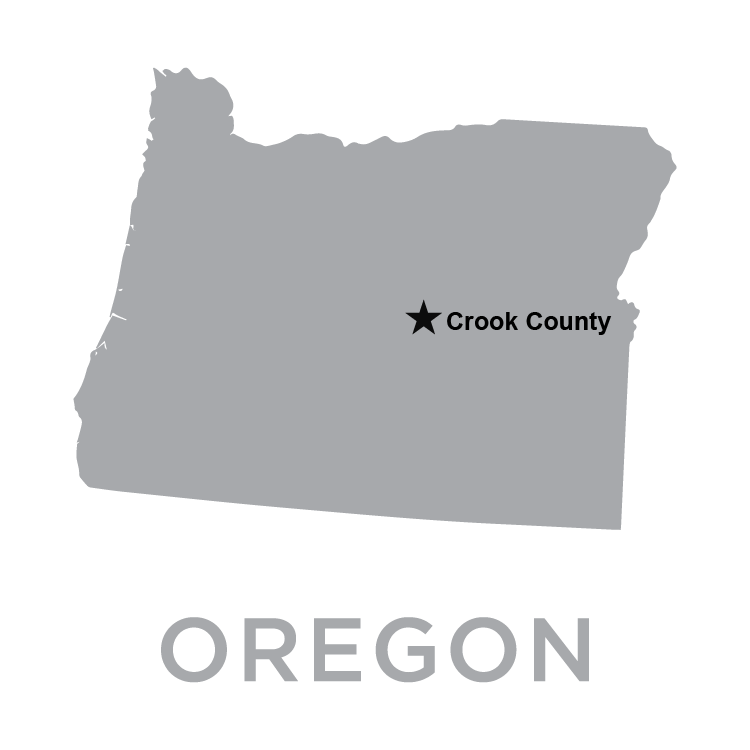
SHIC Monitoring the Situation with Industry Partners and Emphasizes Safety of Pork The Swine Heath Information Center has been coordinating with other swine industry organizations to follow the first detection of H5N1 in a pig in the US. USDA-APHIS confirmed the H5N1 diagnosis from a Crook County, Oregon, backyard farm where a mix of poultry, swine, and other livestock were housed together. Please see the USDA-APHIS press release below.
WASHINGTON, Oct. 30, 2024 – The U.S. Department of Agriculture (USDA) and Oregon state veterinary officials are investigating positive cases of H5N1 in a backyard farming operation in Oregon that has a mix of poultry and livestock, including swine. The Oregon Department of Agriculture announced on Friday, Oct. 25, that poultry on this farm represented the first H5N1 detection in Crook County, Oregon. On Tuesday, Oct. 29, the USDA National Veterinary Services Laboratories also confirmed one of the farm’s five pigs to be infected with H5N1, marking the first detection of H5N1 in swine in the United States. The livestock and poultry on this farm shared water sources, housing, and equipment; in other states, this combination has enabled transmission between species. Although the swine did not display signs of illness, the Oregon Department of Health and USDA tested the five swine for H5N1 out of an abundance of caution and because of the presence of H5N1 in other animals on the premises. The swine were euthanized to facilitate additional diagnostic analysis. Test results were negative for two of the pigs, and test results are still pending for two others. This farm is a non-commercial operation, and the animals were not intended for the commercial food supply. There is no concern about the safety of the nation’s pork supply as a result of this finding. In addition, the farm has been quarantined to prevent further spread of the virus. Other animals, including sheep and goats on the farm, remain under surveillance. USDA’s National Veterinary Services Laboratories (NVSL) has conducted genomic sequencing of virus from the poultry infected on this farm, and that sequencing has not identified any changes to the H5N1 virus that would suggest to USDA and CDC that it is more transmissible to humans, indicating that the current risk to the public remains low. Local public health officials, Oregon Health Authority, Oregon State Veterinarian, Oregon Department of Agriculture, as well as the U.S. Department of Agriculture and U.S. Department of Health and Human Services are coordinating on this investigation and will provide additional updates as they become available. All detections of H5N1 include viral genome sequencing to provide additional information of interest to medical professionals and the research community to improve our understanding of the virus. Genetic sequencing for these samples is underway, though sequencing results may be inconclusive due to low viral levels in the samples. USDA reminds all farmers that strong biosecurity is critical to eradicating this virus and to protecting the health of farmworkers, farmers and their families, livestock and businesses. More information about biosecurity, specifically regarding best practices for farms with multiple species, as well as how to access financial assistance to offset the cost of biosecurity and PPE for farmworkers is available here. Enrollment in these programs can be started with your local Area Veterinarian in Charge (AVIC) or State Animal Health Official. Your nearest USDA Farm Service Agency county office has more information and can also help you enroll.
Read the entire USDA-APHIS release here.

As part of the Swine Health Information Center’s response to the USDA-APHIS-confirmed diagnosis of H5N1 in a backyard pig housed with a mix of poultry and other livestock on a small Oregon farm on October 30, 2024, a webinar titled H5N1 Influenza Risk to US Swine will be held on Wednesday, November 20, 2024, from 11:00 am to 12:30 pm CST.
The recent detection and confirmation of H5N1 in a backyard pig on a multi-species farm by USDA has raised questions regarding the emerging threat and potential risks for commercial swine herds. During the webinar, presenters will provide the latest information on the detection of H5N1 in Oregon, an overview of the risks to swine and knowledge gaps for prevention and preparedness, a review of the impact of H5N1 on the dairy industry, and an overview of experimental studies of H5N1 in pigs.
Confirmed presenters are:
Ryan Scholz, DVM, MPH, State Veterinarian, Oregon Department of Agriculture
Overview of the first detection of H5N1 in a pig on a backyard multispecies farm in Oregon
Montse Torremorell, DVM, PhD, Department Chair and Professor, University of Minnesota
H5N1 risks to swine and knowledge gaps for prevention and preparedness in pigs
Fred Gingrich, DVM, Executive Director, American Association of Bovine Practitioners
H5N1 impact to the dairy industry and update on diagnostic surveillance
Bailey Arruda, DVM, PhD, Research Veterinary Medical Officer, USDA ARS
Update on H5N1 experimental infection studies in pigs
Locke Karriker, DVM, MS, Veterinary Diagnostic and Production Medicine, Iowa State University
Update on aspirin use in pigs
This webinar, hosted by SHIC and the American Association of Swine Veterinarians, is conducted by the Swine Medicine Education Center at Iowa State University.
Highest priority needs for prevention and preparedness of H5N1 infections in the swine industry included
The recent emergence of H5N1 Influenza A clade 2.3.4.4b in dairy animals, coupled with persistent outbreaks in commercial poultry, poses a significant threat to the US swine industry. To summarize the current understanding of H5N1 risk to pigs as well as identify knowledge gaps, the Swine Health Information Center funded a literature review conducted by Dr. Montse Torremorell and colleagues at the University of Minnesota College of Veterinary Medicine. The goal of the literature review completed October 17, 2024, was to help identify highest priority research needs for H5N1 risk to the swine industry, assist in the process of influenza prevention and response planning for pork producers, and provide a summary of the clinical presentation of H5N1 in livestock compared to other known influenza strains.
Given the transmission of H5N1 infection to novel species including livestock, the potential risk of H5 infections in humans, and the epidemiological connections between swine, dairy, and poultry, it is imperative to fully understand the risks to pigs. Developing science-based strategies to prevent H5N1 introduction into swine populations and to contain outbreaks, should they occur, is equally critical.
Read the literature review in full with references here. The following headings highlight topics covered in the review:
H5N1 has caused significant losses in the US poultry industry and has more recently affected the US domestic dairy industry, with infections leading to reduced milk production, changes in milk quality, and occasional respiratory clinical signs. While experimental studies have shown pigs can be infected with H5N1, the specific impact of the 2.3.4.4b clade on pigs remains unknown. The potential for severe consequences to the US pork industry as well as human health is significant.
Experimental infections in pigs have shown variable results, with some studies indicating mild infections and others showing more severe disease. Several factors, such as viral genotype, animal species from which the strain was isolated, and immune status, can influence the severity of H5N1 infections in pigs. Questions surrounding the differences between H5N1 and endemic swine influenza viruses H1 and H3 remain, such as if current protocols for diagnostic surveillance and management of endemic swine influenza can be utilized for H5N1 control and elimination if introduced into commercial swine herds. Direct contact, indirect transmission through fomites, and aerosol transmission are potential routes of H5N1 spread. Enhanced surveillance, improved biosecurity measures, and vaccination strategies are crucial for considering how to best prevent and rapidly control a potential H5N1 infection in pigs.
Results of the literature review confirm studies are needed to assess the clinical signs, viral shedding, and transmission dynamics of H5N1 2.3.4.4b in pigs. Monitoring of pig populations, particularly those with outdoor access or exposure to poultry and dairy cattle, is essential to identify potential H5N1 infections. Literature review authors report research on the survival of H5N1 in various environments, including farm settings and outdoor habitats, is important. Additionally, studies should evaluate whether pre-existing immunity to other influenza viruses or H5N1 vaccines can provide protection against the 2.3.4.4b clade in pigs. For the research needed, validation of diagnostic tests for H5N1 in pigs is necessary to ensure accurate and timely detection.
Addressing these knowledge gaps and implementing effective control measures is crucial to protect the swine industry and public health.

The Swine Health Information Center has partnered with the Foundation for Food & Agriculture Research and the Pork Checkoff to fund a $4 million research program to enhance prevention, preparedness, mitigation, and response capabilities for H5N1 influenza in the US swine herd. H5N1 influenza, an emerging disease identified as a priority for the US pork industry, poses a risk due to ongoing outbreaks in poultry and a growing number of diverse mammalian species susceptible to infection. The unprecedented 2024 H5N1 outbreak impacting dairy herds across the US fuels the urgency for greater understanding and information, along with the recent discovery of the virus in a single backyard pig in Oregon.
Read the full RFP here.
On October 30, 2024, USDA reported the first detection of H5N1 in a pig on a small Oregon backyard farm where pigs were co-housed with poultry and other livestock. Although the farm is a non-commercial operation and the pig was not intended for the commercial food supply, this furthers the concern for potential incursion into US commercial swine herds. Research priorities for H5N1 are designed to further strengthen US swine industry prevention and preparedness as well as inform response efforts should H5N1 be introduced into the commercial swine herd.
SHIC, FFAR, and the National Pork Board invite proposal submissions from qualified researchers for funding consideration to address H5N1 risk to swine research priorities described in the detailed Request for Research Proposals found here along with the instructions for completion and submission, including topic areas of 1) vaccines, 2) clinical presentation, 3) mammary transmission, 4) surveillance, 5) introduction risks, 6) caretakers, 7) biosecurity, 8) pork safety, 9) production impact, and 10) pig movements.
Individual awards are capped at $250,000, however, proposals may exceed cap if sufficient justification is provided. Matching funds are encouraged but not required; the funding cap applies to only those funds requested from SHIC/FFAR/NPB. All projects should strive to have a high impact, show value to pork producers, and have pork industry-wide benefit.
Collaborative projects including the pork industry, allied industry, dairy or poultry industries, academic institutions, and/or public/private partnerships are highly encouraged. Projects demonstrating the most urgent priorities and timeliness of completion, providing the greatest value to pork producers, and showing efficient use of funds will be prioritized for funding. Projects are requested to be completed within a 12-to-18-month period with sufficient justification required for extended project duration.
The deadline for proposal submission is 5:00 PM CT on December 31, 2024. For questions, please contact Dr. Megan Niederwerder at [email protected] or (785)452-8270 or Dr. Lisa Becton at [email protected] or (515)724-9491.
Foundation for Food & Agriculture Research
The Foundation for Food & Agriculture Research (FFAR) builds public-private partnerships to fund bold research addressing big food and agriculture challenges. FFAR was established in the 2014 Farm Bill to increase public agriculture research investments, fill knowledge gaps and complement the U.S. Department Agriculture’s research agenda. FFAR’s model matches federal funding from Congress with private funding, delivering a powerful return on taxpayer investment. Through collaboration and partnerships, FFAR advances actionable science benefiting farmers, consumers and the environment.
Swine Health Information Center
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments. As a conduit of information and research, SHIC encourages sharing of its publications and research. Forward, reprint, and quote SHIC material freely. For more information, visit http://www.swinehealth.org or contact Dr. Megan Niederwerder at [email protected] or Dr. Lisa Becton at [email protected].

The Swine Health Information Center is co-hosting a special session entitled, “Novel Tools and Technologies to Address Emerging Diseases of Swine,” at the 2024 NAPRRS/NC229: International Conference of Swine Viral Diseases from December 8 – 9, 2024, in Chicago. This conference, an annual educational event, brings together swine industry researchers, professionals, and field practitioners to learn about the latest information and research results for emerging viruses, swine health, and biosecurity. SHIC has invited six principal investigators to provide program updates and research results for their SHIC-funded projects.
Coordinated communication and broad dissemination of swine health information to industry stakeholders is part of SHIC’s mission to minimize the impact of emerging disease threats. Working with the NAPRRS/NC229 organizing team offers the opportunity to expand SHIC’s reach by sharing outcomes from work conducted as a result of the Center’s research priorities. The Session will be moderated by SHIC Executive Director Dr. Megan Niederwerder and SHIC Associate Director Dr. Lisa Becton.
Topics and presenters for the SHIC special session at the NAPRRS/NC229 ICSVD include:
NAPRRS/NC229 ICSVD was first held in 2003 and provides a forum to bring together members of the global swine disease community. Though inspired by PRRS-related concerns, the conference has expanded to include other emerging and transboundary viral disease topics in swine. Further information on the conference, including registration and hotel accommodations, can be found here.

The Swine Health Information Center is seeking candidates for the position of Grant and Contract Administrator. Applications in the form of a curriculum vitae with a cover letter describing professional goals and why applicants are uniquely qualified to fill this position should be sent to SHIC Executive Director Dr. Niederwerder at [email protected]. Full consideration will be given to applications received by November 15, 2024. Screening of applicants will start thereafter and continue until the position is filled with an anticipated start date of January 15, 2025. The Grant and Contract Administrator will help the Executive and Associate Directors to support and coordinate operations and business of the Swine Health Information Center. The Grant and Contract Administrator will help to ensure quality research programs and services in line with the mission of the Center. A full job description, including background requirements of the position and further details, is available here. Responsibilities and essential job functions include ensuring the success of SHIC’s grants, partnerships and reporting through organization, documentation and tracking of research projects, as well as seeking funding opportunities to leverage and augment the Center’s operations. This position will maintain compliance with grant provisions, regulations, and reporting requirements. The Administrator will collaborate with external partners, internal stakeholders, and funding agencies to develop new funding opportunities and implement grants from inception to completion. The Administrator will interact closely with the Center Directors, providing administrative support, business coordination, and assisting in efforts to build awareness of the Center and its mission through building and maintaining excellent working relationships. To accomplish this, the position demands critical communication skills and requires a high level of interaction with funding agencies, grantees, research institutions, members of the Swine Health Information Center Board of Directors, industry leaders, working groups, grant review panels, and contract negotiators.

To be responsive to current US swine industry issues and needs related to disease pathogens, the team assembling the Domestic Disease Monitoring Report has developed a survey to gather stakeholder input. Your response to this survey will help improve reports, suggest new features, and provide guidance on additional pathogens to include. SHIC encourages your feedback via this survey, available here. Your response is requested by November 15, 2024. The Swine Health Information Center funds this domestic swine disease surveillance system creating the monthly Domestic Disease Monitoring Report with the goal of sharing information on the activity of endemic and emerging diseases affecting the swine population in the US. The primary objective of the program is to assist veterinarians and producers in making informed decisions on disease prevention, detection, and management.
Initiated in 2019, the Swine Disease Reporting System is a collaborative effort among multiple VDLs to aggregate swine diagnostic data and report it in an intuitive format, describing dynamics of pathogen detection by PCR-based assays over time, specimen, age group, and geographic area. In the beginning, information for one pathogen (PRRSV) from one veterinary diagnostic laboratory was the focus. Since then, five more laboratories have joined the project with six currently participating. Data is collated from the Iowa State University VDL, South Dakota State University ADRDL, University of Minnesota VDL, Kansas State VDL, Ohio ADDL, and Purdue ADDL. Over the last five years, additional pathogens have been added based on recommendations from stakeholder feedback and the SDRS advisory group, which consists of veterinarians and producers across the US swine industry. There are currently nine domestic disease pathogens being monitored through the monthly report, including data on PRRSV (PRRSV-1 and PRRSV-2), PEDV, PDCoV, TGEV, Mycoplasma hyopneumoniae, PCV2, PCV3, and influenza A. Further, SHIC has recently funded the addition of Escherichia coli PCR genotyping to the Domestic Disease Monitoring Reports, allowing for continuous reporting of genotype, virotype, and detection data.
Your input is greatly valued and helps keep the SDRS relevant to current swine industry disease issues. The survey provides opportunities to recommend additional pathogens, laboratories, and features which help increase the value of the report. Thank you for taking the time to complete the SDRS survey!
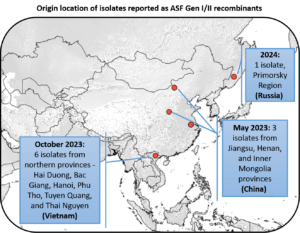
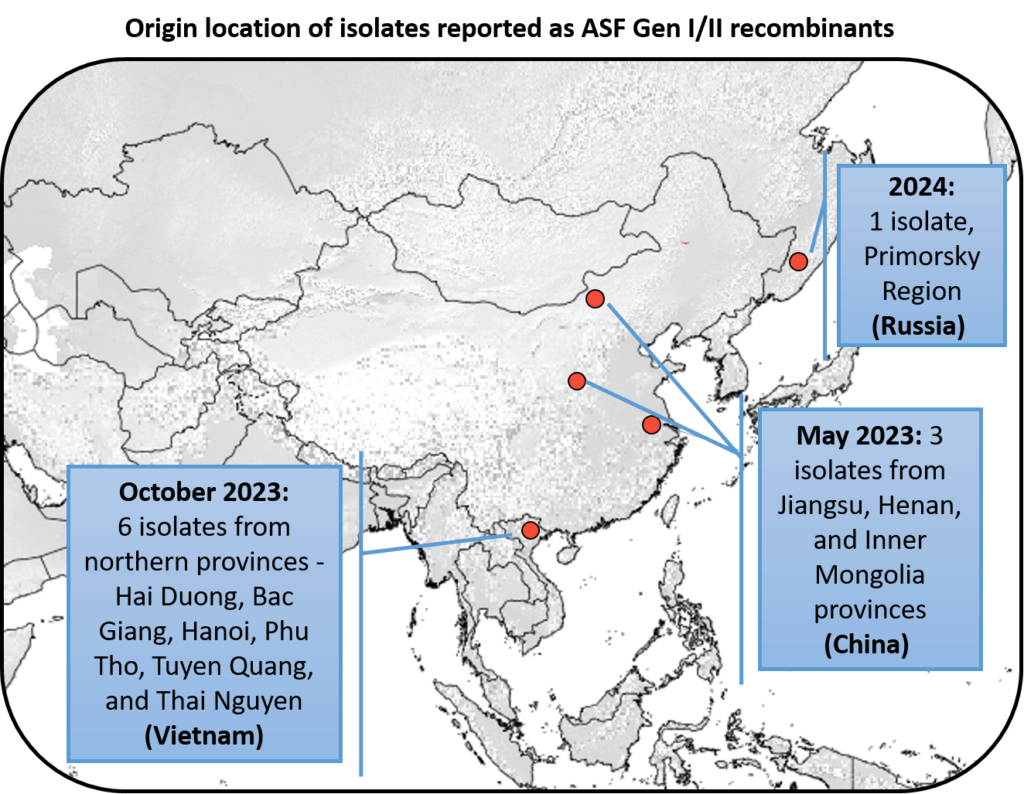
The US swine industry is vulnerable to the introduction of emerging pathogens and routine swine disease monitoring provides early warnings of global challenges that could negatively impact US swine producers. As part of its mission to identify emerging disease threats, SHIC funds Global Swine Disease Monitoring Reports led by Dr. Maria Sol Perez Aguirreburualde at the University of Minnesota. Recent reporting on African swine fever virus strain circulation has highlighted a concerning expansion of the ASFV Recombinant Genotype I/II strains being detected in Asia and now the Russian Far East. Evolving changes in globally circulating ASFV genotypes continue to pose risks for US introduction and inform ongoing prevention and preparedness activities to protect the health of US swine.
Background
Since first being reported in August 2018 on a pig farm near Shenyang, China, 19 additional countries have reported ASF outbreaks in the Asia and Pacific region as of October 2024. The first ASFV genotype occurring in Asia was genotype II, characterized by highly pathogenic strains causing high mortality in domestic pigs. Genotype II is the predominant virus in Europe, Russia, Asia, and the Americas. Ongoing changes in ASFV circulating genotypes are being monitored by the Swine Health Information Center and reported via its Global Swine Disease Monitoring Reports.
Key events altering the ASF epidemiological landscape in Asia
Between 2019 and 2020, there were reports of low pathogenic strains of ASFV genotype II in China and other Asian countries. These low pathogenic genotype II strains were detected during various surveillance activities. Later, in 2021, ASFV genotype I was reported in China which further complicated the epidemiology of the disease in the country and Asia-Pacific region. Prior to this report, ASFV genotype I was only known to be present in Sardinia, Italy, and on the African continent. In 2021, China also reported the discovery of ASF viruses that were a mix between the two genotypes (recombinant viruses).
The recombinant viruses in China were a mix of genetic types, genotypes I and II. These new strains were found in pigs from Jiangsu, Henan, and Inner Mongolia. The viruses were identified as genotype I based on one gene (B646L). However, they showed an unexpected characteristic—they were “HAD-positive” and caused red blood cells to clump together, something not seen in previous genotype I strains in China. This trait is linked to a specific protein, CD2v, encoded by another gene (EP402R). When the researchers sequenced the EP402R gene, they found that it matched genotype II viruses, suggesting a mix between the two genotypes.
In 2023, researchers in Vietnam reported the detection of the recombinant ASFV genotype I and II viruses in domestic pig farms in Northern Vietnam. The recombinant viruses found in Vietnam matched the corresponding sequences of recombinant ASFV I/II strains from China, except for one genetic region, the Central Variable Region. Further molecular analysis of the recombinant strains from Vietnam indicates three possible independent introductions into the country.
ASFV recombinant virus strains reported in Russia
Also in 2023, an ASFV isolate obtained from a domestic pig in the Primorsky Region, a Russian region bordering China, was found to be a recombinant strain. The recombinant genotype I/II virus found in Primosky shares similarities with the recombinant strains from China and has a 99.9% sequence identity, although it does not share identical viral gene sequences. This means the Primosky isolate may be part of a wider transmission network and has undergone minor evolutionary changes as it spread from China. The Primosky recombinant strain presented acute disease in domestic pigs when infected experimentally, which suggests similar characteristics to the Recombinant strains from China.
Timeline of ASFV strain discovery in Asia and the Russian Far East
August 2018 – ASFV emergence in Asia (China) – ASFV genotype II (highly pathogenic strains)
January 2019 – ASFV continues in China and other Asian countries – ASFV genotype II (low pathogenic strains)
December 2020 – ASFV continues in Asia – ASFV genotype II (low pathogenic gene deleted strains)
March 2021 – New outbreak in China – ASFV genotype I (low pathogenic strains)
December 2021 – New outbreak in China – ASFV recombinant genotype I/II
2023 – New outbreaks in Vietnam and Russia – ASFV recombinant genotype I/II
Characteristics of ASFV recombinant strain
Genotype I/II strains from China:
Genotype I/II strains from Vietnam:
Conclusions
The ASFV genotype I/II recombinant strains are circulating in swine and have likely spread to neighboring countries of China, not just Vietnam and Russia. Still, to confirm this assumption, molecular surveillance efforts are lacking in many countries in the region.
The recombinant strains are highly lethal and transmissible in pigs. Based on preliminary evidence, current live-attenuated vaccines based on ASFV genotype II, such as those being implemented in countries such as Vietnam and the Philippines, are most likely not protective against these recombinant genotype I/II strains.
References
1. Zhao, D., Sun, E., Huang, L. et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat Commun 14, 3096 (2023). https://doi.org/10.1038/s41467-023-38868-w
2. Le, V., Nguyen, V., Le, T., Mai, N., Nguyen, V., Than, T….Ambagala, A. (2024). Detection of Recombinant African Swine Fever Virus Strains of p72 Genotypes I and II in Domestic Pigs, Vietnam, 2023. Emerging Infectious Diseases, 30(5), 991-994. https://doi.org/10.3201/eid3005.231775.
3. Sun E, Huang L, Zhang X, Zhang J, Shen D, Zhang Z, Wang Z, Huo H, Wang W, Huangfu H, Wang W, Li F, Liu R, Sun J, Tian Z, Xia W, Guan Y, He X, Zhu Y, Zhao D, Bu Z. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg Microbes Infect. 2021 Dec;10(1):2183-2193. doi: 10.1080/22221751.2021.1999779. PMID: 34709128; PMCID: PMC8635679.
4. Shi K, Liu H, Yin Y, Si H, Long F and Feng S (2022) Molecular Characterization of African Swine Fever Virus From 2019-2020 Outbreaks in Guangxi Province, Southern China. Front. Vet. Sci. 9:912224. doi: 10.3389/fvets.2022.912224
5. Wang X, Wang X, Zhang X, He S, Chen Y, Liu X, Guo C. Genetic Characterization and Variation of African Swine Fever Virus China/GD/2019 Strain in Domestic Pigs. Pathogens. 2022 Jan 14;11(1):97. doi: 10.3390/pathogens11010097. PMID: 35056045; PMCID: PMC8780551.
6. Igolkin, A., Mazloum, A., Zinyakov, N. et al. Detection of the first recombinant African swine fever virus (genotypes I and II) in domestic pigs in Russia. Mol Biol Rep 51, 1011 (2024). https://doi.org/10.1007/s11033-024-09961-0
Stakeholder input for SHIC’s 2025 Plan of work can be submitted here and is requested by November 8, 2024. Input may include topic areas, research priorities, and/or identified industry needs that will focus SHIC’s programmatic and research efforts in 2025, such as an emerging swine disease or an emerging swine health issue. Reference the 2024 Plan of Work here.

This month’s Domestic Swine Disease Monitoring Report includes a bonus page about diagnostic data supporting effective Mycoplasma hyopneumoniae (MHP) control in breeding herds. SDRS reported increased PRRSV positivity in both sow farms and wean-to-finish sites. PRRSV’s overall positivity was above the expected in Iowa, Oklahoma, Indiana, and South Dakota, with Lineages L1C.5 and L1A being predominantly detected. PEDV positivity started to increase at a regional level, with Iowa, Oklahoma, and Missouri beginning to have more positive cases in October. MHP continues with a high percentage of positive submissions in wean-to-finish sites. However, the advisory group reported that most submissions with MHP testing were due to elimination protocols. In the confirmed tissue diagnosis, there were alarms for increased PRRSV, Influenza A virus, and Pasteurella multocida. The podcast broadcasts a talk with Dr. Lisa Becton (Swine Health Information Center) discussing steps to prepare for the potential increase in pathogen activity in the winter and the efforts to eliminate endemic pathogens in the swine industry.

In the November Global Swine Disease Monitoring Report, learn about The Philippines rolling out an ASF vaccine. Their focus is support for backyard pig farms who supply 70-80% of the country’s pork. Legislators and industry groups propose free vaccines to assist these farmers. Elsewhere, the Ukrainian Meat Association urges swift approval of the AVAC ASF live vaccine, while the Ukrainian Pig Breeders Association advises caution until it gains international validation. In Lombardy, Italy, wild boar culling has been intensified along with biosecurity measures against ASF. Over 13,000 wild boars have been culled to date. In India, Mizoram state declared the ASF outbreak a state disaster and submitted a proposal to increase the central government’s cost-share of the outbreak response from 50% to 90%. The Dartford Council in the UK seized 375 lb (170 kg) of illegally imported meat due to ASF risk concerns following an earlier seizure of 3.4 tonnes at Dover – farmers urge more robust post-Brexit border controls. Finally, the Department of Agriculture in The Philippines imposed a temporary import ban on FMD-susceptible animals, products, and by-products from Türkiye due to an FMD outbreak in Kirsehir.

PRRS Cumulative Incidence for MSHMP Beginning July 1, 2009

PEDV Cumulative Incidence for MSHMP Beginning May 1, 2013