
Principal Investigator: Hiep Vu | Institution: University of Nebraska-Lincoln
African swine fever virus (ASFV) is the most devastating viral pathogen which can cause up to 100% mortality in domestic pigs. Swine transportation plays a major role in spreading infectious pathogens. Thus, developing a procedure for effectively disinfecting animal trailers will help reduce viral spreading. A combination of washing, disinfecting, and drying is the most effective method to effectively inactivate pathogens in trailers. However, there are not enough washing facilities to wash all trailers between loads of swine. Therefore, we are interested in testing if it is possible to effectively inactivate ASFV in the presence of organic materials (feces, bedding) through the use of thermal-assisted drying and decontamination (TADD) which commonly operates at the temperature between 63°C and 71°C (1).
With support from SHIC (grant no. 20-071), we found that swabs collected from contaminated trays at all time-points post incubation 54oC and 63oC were positive by PCR, indicating that heat treatment could not eliminate viral genomic DNA. On the other hand, swabs collected from contaminated trays at 5 min post incubation at either 54oC and 63oC were negative by virus isolation assay, indicate that holding ASFV in the presence of feces at 54oC for 5 min was sufficient to inactivate the virus. One major limitation of the previous study was that we used virus isolation as the mean to evaluate virus inactivation. The virus isolation assay might not be sensitive enough to detect virus from samples that have low level of infectious virus.
The primary objective of this amendment was to conduct a pig bioassay to verify if holding ASFV-contaminated feces at 54oC for 10 min would completely inactivate the virus. Consistent with our previous results, we observed in this study that holding ASFV-contaminated feces at 54oC for 10 min resulted in negative virus isolation. However, all four pigs inoculated with the content collected from trays after 10 min incubation at 54oC became infected with the virus, with viral genomic DNA detected in their blood at 5 days post-inoculation. Therefore, incubation of ASFV-contaminated feces at 54oC for 10 min was not enough to completely inactivate the virus.
Objective 1: Test the efficacy of the “Tooth Extraction” protocol for elimination of African Swine Fever Virus (ASFV) from ASF-infected sow farms.
Objective 1a: Using the blood samples from objective 1, compare 5 commercial point of care (POC) assays – 2 rapid antigen-detection tests (aka “quick tests”), POC QT A and QT B and three nucleic acid or polymerase chain reaction (PCR) assays (POC PCR A, B and C) against the standard laboratory-based OIE ASFV PCR (STAND).
Descriptive narrative (M&M):
52 ASFV events were identified by farm caregivers. For each event, whole blood samples were collected from the index sow plus 14 animals in stalls on each side of the index sow (Figure 1F). In total, 764 samples were collected and tested for ASFV DNA by STAND within 24-hours of arrival to the laboratory. For objective 1a, 673 of these samples plus 50 known ASFV negative whole blood samples were tested using the five POC assays according to product instructions. The proportion of positive animals was analyzed as a function of the gestation stall distance from the index sow. Diagnostic sensitivity and specificity were estimated for each POC assay using STAND results as the “true” status.
Discussion of results:
Objective 1: In 17 of the 52 farm identified events (33%), the index sow and 14 neighbor sows were ASFV PCR negative. In 19 (54%) of the 35 events where the index sow was ASFV PCR positive, removal of the index sow and her direct contact neighbors did not remove all ASFV PCR positive sows identified by sampling.
Objective 1a: Compared to STAND, the three POC PCR performed equally with 84-85% diagnostic sensitivity and 95-98% diagnostic specificity on field samples and 98-100% diagnostic specificity on known negative samples.
Compared to STAND, the diagnostic sensitivity and specificity of QT A and QT B were 60% and 88%, and 53% and 74% respectively. Based on known negative samples, both QT tests were 100% diagnostically specific.
Relevance to industry: 1) “Tooth extraction” did not eliminate ASFV from sow farms; 2) ASFV DNA was detected in blood from sows showing no clinical signs; 3) POC tests showed poor diagnostic performance. Limit POC PCR use to clinically ill animals.
African swine fever (ASF) is a foreign animal disease that is a major threat to the U.S. and global pork industries. Thus far, it has remained outside the U.S., but there are ongoing risks of entry due to international trade and travel. Strict regulations limit the importation of pork and pork products to the U.S. from countries known to harbor ASF. However, there have been cases in which ASF has been transmitted in wild boar populations and travelled across regulated borders in Europe. ASF diagnostics are an important tool for managing disease, detecting ASF infections, and preventing further transmissions. Currently, commercial ASF diagnostic tests are only available in Europe, which could limit accessibility to the U.S. if an ASF outbreak were to occur. The goals of this project were to develop and validate an ASF antibody test based on enzyme-linked immunosorbent assays (ELISA), which can be commercialized and accessible in the U.S. We developed two formats of ELISA, indirect (iELISA) and blocking (bELISA), because they can have different advantages in sensitivity (increases early detection) and specificity (reduces false positive misdiagnosis). We evaluated the iELISA and bELISA using over 4000 serum samples collected from swine. Samples were collected from domestic pigs, wild boars from different global regions and in laboratory experiments. We also conducted validation using a standardized panel of ASF serum to evaluate diagnostic performance in three separate ISO-17025 certified laboratories and using ELISA kits from three separate production lots. Through evaluation of data from over 4000 samples, and variability between laboratories and production lots, we concluded that the iELISA and bELISA are both robust ASF antibody tests. They had similar sensitivity, for which they could detect ASF antibodies 8-10 days after infections, and they performed equally well in terms of specificity. Compared to the commercial ASF test in Europe, our ELISAs performed with slightly better sensitivity and similar specificity. Our results are a strong foundation to support ongoing work towards the commercialization of both ASF ELISAs. We aim to follow the procedure to acquire USDA-licensure, after which we would be permitted to manufacture, sell, and distribute the ASF ELISAs as USDA-approved diagnostic products. Providing the pork industry with ASF diagnostic tools is critical for detecting and managing ASF. Outside of the U.S., managing the spread of ASF will protect the global pork industry as well as improve U.S. biosecurity by reducing the risk of ASF entry to the U.S.
The project was focused on helping swine producers in Vietnam to prevent ASF entry into farms and identifying risk factors of virus introduction on the farm. This work was done in collaboration with owners and veterinarians on farms in Vietnam that operate in an endemic area for ASF. The swine farms were infected or did not experience ASF outbreak. We designed surveys to work with interested swine farm owners and veterinarians remotely and translated them into Vietnamese. This helped local producers and biosecurity teams on the farms to work with us to identify gaps on the farms that allowed virus entry. The analysis on the farm biosecurity and our report were provided to the submitter upon completion of the survey. This study and all the confidential reports helped veterinarians or management teams to work on the gaps for biosecurity on the farms in real time. The survey is active as of today and in the event of an outbreak of ASF we will able to help any producers in Vietnam or the US that have an interest to work with us. This is an important project that was supported by SHIC and USDA.
In the absence of effective African swine fever virus (ASFV) vaccines, infection prevention and control through diagnostic testing and quarantine is critical. Early detection and differential diagnosis of ASFV infections increase the chances for successful control of this devastating disease. However, the interpretation of the ASF diagnostic results can be complicated due to the complex epidemiology of the disease, and its unspecific and highly variable clinical presentation, i.e., same strain producing a wide range of clinical forms. The objective of this proposal was to evaluate the performance of ASFV serum/oral fluid indirect ELISA (iELISA) (collaborative work between Innoceleris LLC. and Tetracore Inc.) for surveillance and monitoring of ASFV outbreaks in commercial farms in Vietnam. For this cross-sectional field study, our field team at Hanoi University collected a 398 paired serum/oral fluid samples, individually collected from each animal, which included 100 samples from 34 ASF-acute farms, 98 samples from 47 ASF-chronic farms, and 200 samples from 20 ASF-negative farms. The samples were tested by Tetracore ASFV iELISA and real-time PCR (qPCR). As expected, the detection rate by qPCR (74% serum; 69% oral fluid) was higher than by ELISA (16% serum; 11% oral fluid) in acute farms due to that most of the animals did not seroconvert yet. Contrary, in chronically affected farms, the detection rate of the ELISA was higher (72% serum; 57% oral fluid) than the qPCR (56% serum; 34% oral fluid). However, when we combined both qPCR and ELISA, the detection rate of ASFV positive animal increased in acute (75% serum; 74% oral fluid) and particularly in chronic farms (85% serum; 74% oral fluid). All serum samples from negative farms were negative by both ELISA and qPCR (100% diagnostic specificity) while, for oral fluids, we obtained 100% and 99% diagnostic specificity for qPCR and ELISA, respectively. The high diagnostic specificity of the tests is particularly important for ASF surveillance. Absence of false positives avoid false alarms and disruption in production, and lack of confidence in the tests/surveillance system. This unprecedented study show that there are no single best diagnostic approach for ASFV surveillance and demonstrate that the combined use of the Tetracore qPCR and indirect ELISA tests and serum/oral fluid sampling, increase efficiency of ASF disease surveillance.
For additional information: [email protected]; [email protected].
Objective #1
The objective of this portion of the study was to determine whether rodents trapped in and around ASF-infected farms harbored ASFV (African Swine Fever virus), and if so, which animal samples are the best ones from which to detect viral infection.
Five commercial farms that had recently experienced ASF outbreaks were identified. Farms were located across 4 provinces in northern Vietnam and ranged in size from 26-851 pigs. Biosecurity procedures varied among the farms; 2 farms featured closed housing, 1 open housing, and 2 combined closed and open housing.
Four of the 5 farms had experienced ASF outbreaks in the spring and summer of 2020, while the 5th farm last broke in May 2019. Pigs from 4/5 farms were sampled in July 2020, with pigs testing positive on 3/4 farms (PCR testing of blood or necropsy specimens). The farm testing negative had experienced their last outbreak approximately 1 year prior; the farm not tested was considered ASF-positive by their herd veterinarian but no testing results were available.
Live traps were placed nightly at each study farm, outside facilities at entry points, and inside facilities near feed sources. Trapping continued at each farm for 10 to 36 days or until 10 rats were obtained. A total of 34 rats were obtained among the 5 farms, with 2 to 10 captured per farm. Rats were euthanized on the farm, stored in freezers, and transported to VNUA for necropsy and PCR testing.
Spleen, front paw, and feces were tested from each rat (n=27) obtained from the 3 confirmed ASFV-positive pig farms; all samples were negative for ASFV. Samples obtained from the other 2 study farms (blood, spleen, liver, lung, ileum, and front paw from rats from 1 farm (n=5); spleen, front paw and feces from the other (n=2)) were negative for ASFV as well.
Samples from rats in and around farms undergoing active ASF outbreaks were negative for ASF across several different sample types (examining biological as well as mechanical vector potential). This work suggests that rats (and presumably similar rodents) do not serve as important vectors for ASFV.
Objective #2
Building on the negative results from Experiment 1, the second experiment examined whether rats were susceptible to challenge with ASFV, and if so whether they were able to transmit the virus to susceptible rodents.
Rats were randomly assigned to 1 of 6 ASF challenge cages (n=6 each). In each challenge cage, 3 rats were challenged with a dose of 105 50% hemadsorption doses (HAD50) ASF either intraperitoneally or orally (day 0), while 3 additional rats in each cage served as susceptible contacts. One cage of 9 rats served as non-inoculated controls. Body temperatures and clinical signs were recorded 3 times/day for each animal.
On each of days 7, 14, and 21, rats in 2 cages (1 oral inoculation, 1 intraperitoneal) were euthanized and samples tested for ASFV. Three rats from the non-inoculated control cage were similarly euthanized, sampled and tested each of these days.
Clinical signs were not present in any rats during the observation periods. There were no differences between the body temperature of control and inoculated rats, although temperatures of all rats (control, inoculated, and contacts) climbed during the second week of the experiment, then fell back to baseline.
At each sampling point, blood, spleen, liver, lung, and ileum were tested via PCR for ASFV. None of the samples were positive for ASFV. Serum analysis using an ELISA test to detect antibodies were negative for all rats at all collection points. Despite robust challenges both intraperitoneally and orally, rats were not observed to become ill from nor infected with ASFV out to an incubation period of 21 days.
For more information, contact Dr. Russ Daly, [email protected]
African swine fever (ASF) is a devastating viral disease of domestic pigs with mortality rate that can approach 100%. ASF control mainly relies on strict biosecurity that involves movement restriction, quarantine, and compulsive depopulation of affected herds. Rapid and reliable detection of ASFV infected pigs is critical for the control of ASFV. One desirable property of a diagnostic tests is the capacity to detect viral infection, especially during the incubation time, when the infected animals have not displayed any clinical signs. In this study, we evaluated performance of three pen-side tests for ASFV detection: one PCR test for detection of viral genomic DNA and two lateral flow tests for detection of viral antigens.
The first objective was to determine the time from infection to the earliest detection. Ten pigs were experimentally infected with an ASFV strain. Whole blood and oral swab samples were alternatively collected from five pigs every other day post-infection (dpi) and tested with the three pen-side tests. The pen-side PCR test detected infected pigs starting from 2 dpi when using whole blood and 3 dpi when using oral swabs. It consistently detected infection until the end of the study (10dpi). The antigen test detected infected pigs starting from 3 dpi and no longer detected infection at 10 dpi when using whole blood. The antigen test did not work well when tested with oral swabs. Compared with the reference laboratory real-time PCR test, the pen-side PCR test exhibited 97.8% sensitivity and 100% specificity. The antigen had 100 specificity but only 47.8% sensitivity, mainly because it failed to detect infection from samples collected early or late after infection.
The second objective was to evaluate the diagnostic performance of the tests with samples collected from the field. Whole blood and oral swabs were collected from 205 pigs: 34 positives and 171 negatives as determined by the reference laboratory real-time PCR. All pen-side tests had 100% specificity, regardless of the sample types tested. The sensitivity of the pen-side PCR test was 88.2% and 70.4%, respectively when tested with whole blood and oral swab. The sensitivity of the antigen test 50% and 11.11% respectively when tested with whole blood and oral swabs.
In summary, the results of this study show that the PCR pen-side test has better performance than the antigen test as it can detect infected pigs earlier and for a longer duration after infection than the antigen test. Additionally, the pen-side PCR test works with both whole blood and oral swabs while the antigen test work with only whole blood.
Our overall objective was to determine the risk of introducing African Swine Fever (ASF) to a sow farm as a result of semen movement from apparently healthy boar studs located in an ASF disease control area. We performed a proactive risk assessment (RA) that looked at the potential risk of semen movements during an outbreak. We established the ASF Boar Semen RA workgroup (WG) whose members included 19 boar stud subject matter experts (SME), six pork producer representatives, 12 federal / state agency veterinarians, and 16 academics. We worked closely with the WG to answer these questions.
The ten potential entry pathways are people, feed, water, geographic and/or aerosol transmission, fomites (such as tools, equipment, vehicles), mortality management, domestic animals (such as dogs, cats, replacement boars), biological materials (such as medicines and vaccines), insects/ticks, and wildlife.
As a result of our WG meetings and studying the findings from both published scientific reports and data from outbreaks in Vietnam, we have evaluated each of the pathways and assigned them a likelihood rank that ranges from negligible to high, as described in Table 1.

The estimated likelihood of ASFv infection of a boar stud operation in a Control Area due to water was negligible, as long as no surface water is being utilized in the boar stud operation.
The likelihood of ASFv introduction was negligible to low for feed, insects/arthropods, and wildlife (including infected feral pigs), as long as boar studs continue their standard biosecurity practices such as tandem feed bins, insect control, indoor housing, and double fencing.
The likelihood of ASFv introduction was low for people, fomites, domestic animals (including replacement boars), and biological materials, as long as boar studs continue requirements and procedures including but not limited to shower-in/shower-out people entry with downtime from other pigs, decontamination and disinfection for materials entering the stud, and housing of replacement boars in isolation barns away from the boar stud and lab.
It is very important to note for seven potential entry pathways of ASF infection (people, feed, fomites, animals, insects/arthropods, wildlife, and domestic animals), there are suggested Enhanced Biosecurity Recommendations (EBRs) in the Secure Pork Supply (SPS) plan that, if followed and done correctly, are critical to lowering the risk of ASF infection. Therefore, following all EBRs was assumed to occur when these ratings were made, and examples of these biosecurity practices have been given above (for example, shower-in/shower-out). On top of the EBRs in the SPS plan, the WG proposed putting into place targeted EBRs to further reduce the risk of ASF infection. When the WG decided these targeted EBRs were feasible by the vast majority of boar studs in the US swine industry, these protective actions were included in the estimates of the likelihood ratings. The estimated likelihoods of ASFv infection via the ten potential pathways are summarized in Table 2.
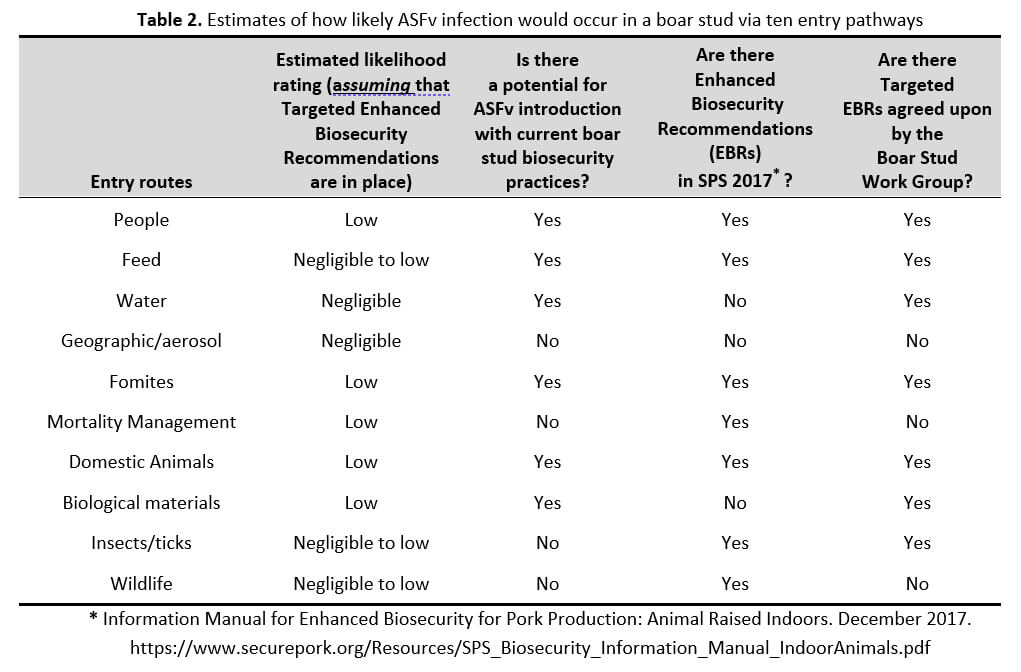
This proactive risk assessment is an evolving, product-specific risk assessment that will be reviewed before distribution to the swine industry, state animal health officials, and the U.S. Department of Agriculture Animal and Plant Health Inspection Service (USDA: APHIS). It will be reviewed and updated as necessary before and during an ASF outbreak to incorporate the latest scientific information and preventive measures. If the Incident Command System (ICS) is activated in response to an ASF outbreak, Incident Command staff will review this risk assessment to assess industry requests for movement of liquid, cooled boar semen from a boar stud in a control area.
African swine fever virus (ASFV) is the most devastating viral pathogen which can cause up to 100% mortality in domestic pigs. Currently, the virus is affecting many swine producing countries, leading to the approximately 25% reduction of global swine population. Swine transportation plays a major role in spreading infectious pathogens. Thus, developing a procedure for effectively disinfecting animal trailers will help reduce viral spreading. The most effective method to effectively inactivate pathogens in transportation trails is a combination of washing, disinfecting and drying. However, there are not enough washing facilities to wash all trailers between loads of swine. Therefore, we are interested in testing if it is possible to effectively inactivate ASFV in the presence of organic materials (feces, bedding) through the use of thermal-assisted drying and decontamination (TADD) which commonly operates at the temperature between 63°C and 71°C (Dee et al., 2005).
The objective of this project is to determine the optimal baking time and temperature required to completely inactivate ASFV in aluminum surface contaminated swine feces. We tested the inactivation efficiency of contaminated trays under 3 conditions:
Two different methods were used to evaluate the efficiency of the treatments: PCR to detect of viral genomic DNA and virus isolation to detect infectious virus.
In the first condition, swabs collected from contaminated trays at all time-points post incubation at 54oC and 63oC were positive by PCR, indicating that heat treatment could not eliminate viral genomic DNA. On the other hand, swabs collected from contaminated tray at 5 min post incubation at either 54oC or 63oC were negative by virus isolation, indicate that holding ASFV in the presence of feces at 54oC for 5 min is sufficient to inactivate the virus.
In the second and third conditions, only two swabs collected after washing were positive by PCR at high Ct value (e.g. 37.03). These swabs were negative by for virus isolation. Thus, under the conditions of this study, power washing of the trays with water at room temperature was efficiently remove contaminated material from the trays.
Collectively, results obtained from this research provide valuate information for the develop effective sanitation protocols to disinfect animal trailers to reduce the spreading of ASFV.
Principal Investigator: Aruna Ambagala | Institution: Canadian Food Inspection Agency – National Centre for Foreign Animal Diseases (CFIA-NCFAD)
Oral (rope) fluid is an easily collected group sample that can be tested at veterinary diagnostic laboratories for the presence of bacterial and viral pathogens circulating in pig herds. It is widely used to detect endemic pathogens in swine such as PRRSV, PCV2, SIV, M. hyopneumoniae etc. In the event of an ASF outbreak in US or Canada, it’s important to maintain zoning and compartmentalization requirements to maintain pork trade from unaffected areas, while testing and eliminating affected animals. Oral fluid is a sample type that can be helpful in active surveillance to screen swine herds for early detection of ASF, particularly in that situation. Previously using experimentally infected pigs we have shown that swine oral fluids can be used to detect African swine fever in commercial size pig pens. The objective of the present study was to conduct a field evaluation of oral fluids in the ASF endemic country of Vietnam for early detection of ASF virus, during active outbreaks.
The project was significantly delayed due to travel restrictions and lockdowns during the COVID-19 pandemic. Despite challenges, between 2021 and 2023, we successfully conducted three independent studies. In all three studies, we sourced pigs from family farms diagnosed with ASF and willing to contribute to the study. One infected farm at a time was evaluated in each study. Upon confirmation of ASF in the farm, apparently healthy pigs from the infected farm were purchased and transferred to a clean study farm and assigned to pens (varying pen sizes in each study). In each pen, ropes were hung, pigs were allowed to chew the ropes for 30 minutes and oral fluid was collected on a daily basis until the end of study or till the pigs refused to chew the ropes. Whole blood and oropharyngeal swabs were collected from individual pigs either every other day or daily depending on the study. Rectal temperatures of the pigs were also measured and clinical signs were monitored on a daily basis. When the pigs developed fever and other clinical signs of ASF, their blood samples were tested to confirm/rule out ASF. Each study was conducted for a different period of time (depending on the clinical picture observed and the disease progression) to maximize the number of oral fluid and paired whole blood samples collected. Samples were tested according to the NCFAD standard protocol for testing oral fluid and whole blood for ASF.
Study 1 was conducted in 2021 December. A total of 94 clinically healthy pigs were collected from an ASF positive family farm, 19 days following the original detection and transferred to a clean study farm and assigned into pens A, B, C & D. Samples were collected as described earlier, up to 30 days. The results from Study 1 showed ASFV infection in one pig in pen B, with slow spread to other pens. In pen B, ASFV was detected in whole blood on day 3 of sampling. Oral fluids from Pen B tested positive for ASFV starting on day 6, i.e., within 3 days of detecting the first pig with viremia. In pen A, the first viremia and first oral fluid detection happened on 14 day of sampling. In pens C and D, viremia was first detected on day 24 of sampling and ASF genome detected in oral fluids within 2 days, i.e., on day 26 of sampling.
Study 2 was conducted in February 2023. For this study, 117 apparently healthy pigs were purchased from an infected farm 2 days after confirmation of infection and transferred to a clean study farm. One day later, 60 additional pigs from the infected farm were bought and transferred to the same study farm. The first 117 pigs were assigned to pens A-D and the additional 60 pigs were assigned to pens E-H. During transportation (2 days), it was noticed that some pigs developed diarrhea. Upon arrival, whole blood samples were tested for ASF and a large number of pigs (20-74% per pen) tested positive for ASFV genome. ASFV genome was also detected at high levels in oral fluid samples collected from all the pens starting with day 1 of sampling. Despite viremia, the pigs showed no ASF-specific clinical signs until 3 days post sampling when the first pig died (Pen D).
Study 3 consisted of 104 pigs and was conducted in April 2023. Apparently-healthy pigs were purchased 3 days after the confirmation of ASF in the farm, transferred to a study farm, and were assigned to 4 pens (A-D). Whole blood samples from two pigs in Pen A tested positive for ASFV on day 4 of sampling. Oral fluid from the same pen tested positive for ASFV genome on day 6 of sampling, 2 days following the initial detection of viremia in the pen. Oral fluid detection continued till the end of study. In Pen C, ASF was detected in whole blood on day 7, and the first detection in oral fluids on day 9 of sampling. In Pens B and D, both initial viremia and oral fluid detection occurred on day 1 of sampling and continued thereafter. To summarize Study 3, detection of ASF DNA in oral fluids occurred within 0-2 days of the first detection of viremia in the pens and continued through the end of sample collection.
Based on the overall data of this project, ASFV DNA can be detected in oral fluids within 0-3 days of the initial detection of viremia in the pen. This is consistent with the results from our previous study on experimentally inoculated animals under experimental conditions. If the viral load is low in the pen, it may take up to 3 days to detect in oral fluids. This work confirms and further validates oral fluids as a reliable aggregate sample for screening swine herds for early detection of ASF.
Contact Information:
Aruna Ambagala, BVSc, MSc, PhD
Research Scientist
Head-Mammalian Diseases Unit
WOAH Reference Laboratory for CSF and ASF
CFIA-National Centre for Foreign Animal Disease
1015, Arlington Street, Winnipeg, MB R3E 3M4, Canada
Tel. 204-789-2013 (Office); 204-789-2089 (Lab)
Email: [email protected]
Copyright 2024 | Swinehealth.org | Website by Heartland Marketing Group