
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments.

Join SHIC Associate Director Dr. Lisa Becton and host Barb Determan as they review how to access and use SHIC information including disease monitoring reports, research results, and more.

Please click here to provide your input for SHIC’s 2026 Plan of Work before our December 1, 2025, deadline! The 2026 Plan of Work is currently under development and will be built around SHIC’s five strategic priorities: 1) improving swine health information, 2) monitoring and mitigating risks to swine health, 3) responding to emerging disease, 4) surveillance and discovery of emerging disease, and 5) swine disease matrices. Find the 2025 Plan of Work here for reference.
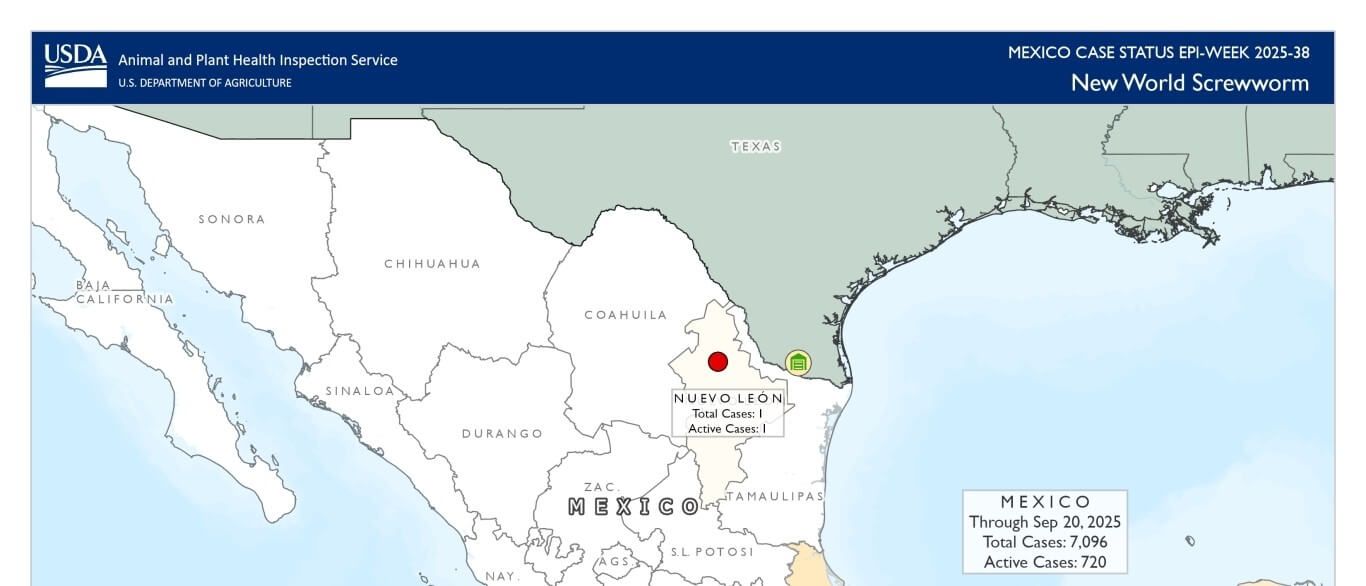
On September 21, 2025, USDA reported the presence of New World Screwworm in Sabinas Hidalgo, located in the state of Nuevo León, Mexico, less than 70 miles from the US-Mexico border. USDA said this is the northernmost detection of NWS during the current outbreak and, consequently, the most threatening to the US livestock industry. The previous northernmost case was reported on July 9, 2025, in Veracruz, approximately 370 miles further south of Sabinas Hidalgo. On September 21, 2025, NWS was detected in an eight-month-old heifer that had been transported to Sabinas Hidalgo from a region in southern Mexico with known active NWS cases. Identifying and responding to NWS becomes essential as the pest nears the US border.
NWS larvae, commonly known as maggots, can infest livestock and other warm-blooded animals, including humans. They most often enter through an open wound, some as small as the size of a tick bite, and feed on the animal’s living flesh. They can also gain access to the host through body openings including the nose and mouth. The name screwworm refers to the maggots’ feeding behavior as they burrow into the wound, feeding as they go, like a screw going into wood. NWS maggots cause damage by tearing at the host animals’ tissue with sharp mouth hooks. The wound can become larger and deepen as more maggots hatch and feed on living tissue. As a result, NWS can cause serious, often deadly, damage to the animal, as well as a great deal of pain, becoming an animal welfare issue. Adult screwworm flies are about the size of a common housefly or slightly larger. They have orange eyes, a metallic blue or green body, and three dark stripes along their backs.
Surveillance Information
USDA provides guidance for detection and surveillance of NWS. Watch for:

If suspected clinical signs are noted, contact your herd veterinarian immediately as NWS is a reportable disease to the State Animal Health Official and the USDA federal veterinary authorities. The process for reporting is provided by USDA in their publication, Foreign Animal Disease Investigation Guide: New World Screwworm. Additional information is included in the USDA publication, Standard Operating Procedure for Possible Detections of New World Screwworm in Animals.
Historic Perspective and Control Strategies
While NWS is currently not in the US, other than a single human case in August 2025 due to travel to affected areas, NWS has been an issue for US livestock producers since at least 1842 according to a USDA publication, New World Screwworm Ready Reference Guide – Historical Economic Impact. The need to prevent NWS infestation in the US and its cost to the livestock sector and overall US economy is clear.
During a SHIC/AASV webinar on NWS conducted on June 13, 2025, Dr. Cody Egnor, veterinary medical officer at USDA, addressed expected control strategies. USDA has actively focused on the use of Sterile Insect Technique to reduce and eliminate the flies in affected locations. This method involves the release of sterile flies into the wild population to reduce the reproductive capacity of NWS flies and eventually eliminate them from the environment. Prevention activities include robust regulatory controls, active field surveillance for myiasis, sterile fly release, and stakeholder engagement. USDA’s preparedness and response activities are contained in the Disease Response Strategy – New World Screwworm Myiasis.
Currently, there are no labeled products approved for treatment of NWS myiasis in swine. However, USDA and FDA are working together to evaluate emergency and conditional use of specific products in livestock species. Additional information from the FDA regarding NWS and treatment guidance for veterinarians can be found in the document, Animal Drugs for New World Screwworm.

The Swine Health Information Center, along with the Foundation for Food & Agriculture Research and the Pork Checkoff, launched the Wean-to-Harvest Biosecurity Research Program in the fall of 2022. Goals of the research program were to investigate cost-effective, innovative technologies, protocols, and ideas to enhance biosecurity implementation during the wean-to-harvest phases of swine production. Results received to date provide opportunities for US pork producers to understand potential risks, identify steps to prevent PRRS transmission, and make changes to immediately enhance their herd biosecurity as the fall respiratory disease season approaches.
At wean-to-harvest sites, biosecurity practices remain inconsistent and less rigorously enforced than at sow or boar stud sites, increasing the risk for disease introduction and transmission. The Wean-to-Harvest Biosecurity Research Program addresses several key biosecurity areas, including bioexclusion (keeping disease off the farm), biocontainment (keeping disease on the farm after an outbreak to lessen risk to neighbors), and transportation biosecurity. A total of 24 projects have been funded through this program to provide a comprehensive approach to advancing biosecurity of US farms. The Wean-to-Harvest Research Program reflects SHIC’s responsiveness to an identified swine health vulnerability and its collaborative efforts to leverage producer Checkoff dollars to safeguard the health of the US swine herd.
Projects focusing on disease introduction risks, using PRRSV as one of the targeted pathogens at wean-to-harvest sites, have identified key knowledge and tools that producers and veterinarians can apply at the farm today to combat PRRS transmission and introduction into the herd. Outcomes relevant for fall biosecurity are summarized here from two projects, including an industry-wide assessment of bioexclusion practices led by Dr. Gustavo Silva and an assessment of manure pumping effects on disease onset led by Dr. Daniel Linhares, both at Iowa State University.
Investigations of factors influencing the risks of disease introduction and transmission at wean-to-harvest sites and during transportation have provided key takeaways informing biosecurity enhancement for PRRS management and control:
Read more about these projects by searching for 23-029 and 23-031 here.
PRRS remains an industry-wide swine health challenge, and as emerging variants continue to be detected in the US, identifying risks and implementing biosecurity steps to prevent the introduction and spread of the virus are critically important. Proactively enhancing wean-to-harvest biosecurity can assist in the control of emerging and endemic diseases across the US pork industry.
While the Wean-to-Harvest Biosecurity Research Program is no longer accepting new applications, many projects are ongoing, and outcomes will be published as soon as they become available for stakeholder awareness and application on-farm. SHIC continues to invite research proposal submissions that address its five strategic pillars and priorities outlined within the 2025 Plan of Work, spanning multiple facets of swine health. Targeted research on key priority areas, such as biosecurity for wean-to-harvest production, provide knowledge for identifying emerging threats and solutions to reduce impact on pork producers.
Foundation for Food & Agriculture Research
The Foundation for Food & Agriculture Research (FFAR) builds public-private partnerships to fund bold research addressing big food and agriculture challenges. FFAR was established in the 2014 Farm Bill to increase public agriculture research investments, fill knowledge gaps and complement the U.S. Department Agriculture’s research agenda. FFAR’s model matches federal funding from Congress with private funding, delivering a powerful return on taxpayer investment. Through collaboration and partnerships, FFAR advances actionable science benefiting farmers, consumers and the environment.
Swine Health Information Center
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments. As a conduit of information and research, SHIC encourages sharing of its publications and research. Forward, reprint, and quote SHIC material freely. For more information, visit http://www.swinehealth.org or contact Dr. Megan Niederwerder at [email protected] or Dr. Lisa Becton at [email protected].

The Swine Health Information Center funded a project to generate a data-driven swine disease index through monitoring swine pathogen activity using confirmed tissue-based diagnoses from Iowa State University Veterinary Diagnostic Laboratory. This initiative aimed to provide a transparent, automated, and reproducible method to help veterinarians, producers, and stakeholders prioritize disease threats based on real-world diagnostic data. Led by PhD Candidate Guilherme Cezar under the supervision of principal investigators Drs. Giovani Trevisan and Daniel Linhares, the resulting disease index monitors swine pathogen activity and identifies emerging threats. In addition, the index can be adapted and integrated into the SHIC-funded Swine Disease Reporting System for continuous monitoring.
Find the industry summary for Swine Health Information Center project #24-016 here.
To build the swine disease index, 59,950 ISU-VDL porcine cases from 2020 to 2024 were utilized. Four key factors were considered:
These factors were weighted and combined into a single score for each disease, updated weekly for the ongoing year, and displayed in an interactive Power BI dashboard that will be housed on the SDRS website. Investigators anticipate the Power BI Dashboard will be available in early 2026 and will display annual disease index data. The dataset used was annotated by diagnostic codes, farm type, geographical data, and accession IDs. Four normalized variables were used to build the index: disease occurrence, co-diagnoses, geographic spread, and Early Aberration Reporting System (EARS) alarms. These measures were weighted using an R-based function and combined into a single scale ranging from 0.01 to 1. Statistical validation—using resampling, Euclidean, and Manhattan distance models—ensured robust consistency across years.
Results confirmed that PRRSV and Streptococcus suis remain the top two-ranked pathogens, demonstrating their high activity in the US swine industry. The system also detected emerging pathogens’ activity in 2024, including porcine sapovirus and porcine astrovirus, while PCV2 showed a notable decline. The dashboard allows users to track disease trends and compare rankings year-to-year, supporting real time decision-making. The index was validated using modeling to assess year-to-year consistency and detect emerging or declining disease trends. Bootstrap resampling (500 iterations) generated 95% confidence intervals for index predictions, excluding EARS due to model limitations. The interactive Power BI dashboard provides real-time visualization and weekly index updates which can be monitored by the SDRS team, and ongoing changes communicated to the industryKey findings revealed high stability in pathogen indexes across years, with a Spearman correlation of 0.92. PRRSV and Streptococcus suis consistently ranked as top burdens, underscoring their persistent importance. However, the method also registered new priorities: porcine sapovirus and astrovirus after they had an increased number of cases in 2022 and 2023, respectively, emerged as more significant endemic pathogens. In contrast, PCV2, traditionally considered a dominant pathogen, fell out of the top 10 by 2024. This decline may reflect improved herd immunity, vaccination, or biosecurity interventions.
Although infectious diseases dominated the rankings, the system also captured nutritional and toxicological diagnoses, which—while less common—introduce diversity to the surveillance profile. Their variability and occasional abrupt ranking shifts highlight the utility of keeping such categories under close observation for early detection of non-infectious threats.
The disease index’s strength lies not only in its reproducibility but also in its adaptability. Its integration into a Power BI dashboard monitored internally by SDRS staff provides a real-time, visually accessible tool, updated weekly, that supports veterinarians, producers, and industry stakeholders in quickly identifying emerging disease threats and allocating resources efficiently. By combining multifactorial epidemiological variables rather than relying on occurrence alone, the index captures both pathogen prevalence and broader dynamics such as geographic spread and co-disease patterns.
Overall, the index presents a scalable and transparent foundation for swine disease prioritization, enabling continuous, longitudinal monitoring that differentiates between stable endemic pathogens and volatile or emerging threats. Its validation across multiple statistical models ensures consistency while maintaining sensitivity to dynamic changes. Moving forward, investigators recommend integrating the framework into SDRS, refining weighting algorithms, and considering extension of the concept to other livestock systems to further enhance animal health security. By combining epidemiological rigor with automated analytics, this study delivers a timely and flexible tool that strengthens swine health surveillance and informs science-based disease control strategies.
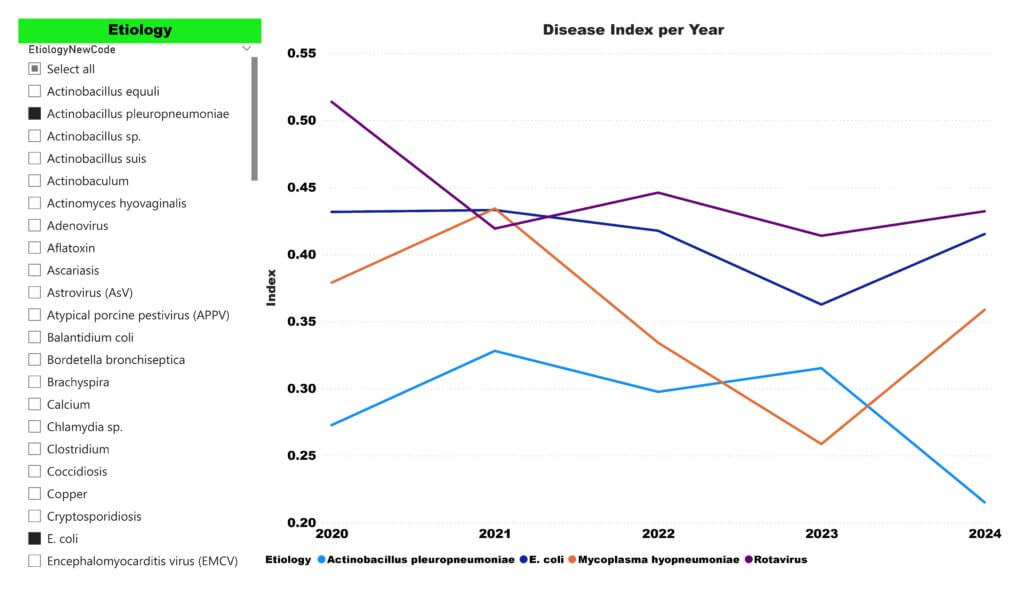
The recent addition of E. coli PCR genotyping to the SHIC-funded Domestic Swine Disease Monitoring Report continues to show support towards evidence-based herd health decision-making across the US pork industry. E. coli is a significant pathogen in swine, most frequently associated with neonatal and post-weaning diarrhea. With the inclusion of E.coli in the Swine Disease Reporting System published in monthly SHIC communications, producers, veterinarians, and researchers now gain access to real-time surveillance data on one of the most important and complex pathogens affecting pigs today.
Find the industry summary for Swine Health Information Center project #24-017 here.
Dr. Giovani Trevisan, co-principal investigator with Dr. Daniel Linhares for the SDRS at Iowa State University, explained, “The decision to add E. coli was in response to requests from the SDRS Advisory Board and multiple stakeholders in the industry. E. coli is a significant and complex pathogen affecting swine health, responsible for a wide range of diseases. The SHIC Swine Bacterial Disease Matrix ranks E. coli as the 5th most prioritized and clinically important bacterium for the US pork industry, with a score of 21.7, second only by a narrow margin to the 21.8 score for M. hyopneumoniae. That ranking underscores how concerning it truly is.”
Understanding and Reporting on this Significant Pathogen
E. coli remains a challenge for bacterial disease management within swine populations because of its diverse pathotypes and its capacity to cause severe economic loss. Its clinical manifestations range from post-weaning diarrhea to edema disease, conditions that can undermine welfare while also inflating production costs. For years, stakeholders have recognized the value of broader surveillance of this pathogen. With the integration of E. coli into the SHIC monthly reports, this information is now available for producers and veterinarians to use in the management of E. coli challenges.
According to Dr. Trevisan, building the infrastructure necessary to add E. coli to SDRS was a complex process. “The efforts to add E. coli started in February 2024, with a learning journey on how to report a complex pathogen such as E. coli. The data organization and hub development began shortly after SHIC funded the proposal. What became clear was the need for extensive standardization across participating veterinary diagnostic labs.”
This process of data organization reflected the inherent complexity of the pathogen. Unlike many viral targets characterized in SDRS, E. coli behaves both as a benign commensal organism and as a highly pathogenic agent. To interpret results meaningfully, the surveillance network needed to account for genotypes, virotypes, and virulence factors that define pathogenic potential. Harmonizing submissions across laboratories to achieve this level of granularity requires significant cross-institutional collaboration.
The inclusion of E. coli data in SDRS now provides unprecedented insight into bacterial disease dynamics on a national scale. As Dr. Trevisan described, “With this expansion, producers, practitioners, researchers, and other stakeholders have access to centralized, near-real-time data on testing results, including E. coli genotyping PCR targets, virotypes, and pathotypes being detected. The advantages are multifaceted.”
Insights Gained in Developing the E. coli Report
The process of integrating E. coli into SDRS was championed and became part of the master of science degree thesis of Elisa de Conti and has already yielded important learnings that further highlight the complexity of this pathogen. Dr. Trevisan observed, “We learned that E. coli’s complex ecology demands a reporting system that is both comprehensive and flexible. Reporting must occur at the sample level, understanding what was detected within each isolate or sample submitted for genotyping PCR. This approach enabled us to classify submissions as potentially pathogenic or not, which is fundamental for epidemiological interpretation.”
Several findings have surfaced during this preparation. Approximately one-third of tested samples lacked pathogenic potential, demonstrating the need for nuanced reporting. Since 2017, detections of fimbriae have shifted, with F18 steadily increasing while K88 (F4) declined. Among virotypes, the combination of F18:LT:STa:STb:Stx2e emerged as the most frequently found across multiple states. Specifically, this F18:LT:STa:STb:Stx2e virotype was the most frequently detected in 2024, representing 37.8% (389/1,030) of the virotypes. Perhaps most notably, since 2021, hybrid ETEC/STEC pathotypes have been detected more frequently than classic ETEC strains, signaling a significant epidemiological shift in circulating E. coli populations.
These trends provide actionable intelligence. For veterinarians, recognizing a rising prevalence of certain virulence factors can inform vaccine and treatment strategies tailored to herd needs. For the broader industry, an understanding of regional patterns supports collaborative approaches to mitigation and control.
The benefits are highly practical. Diagnostic decision-making can be enhanced, especially considering emerging pathotypes such as the hybrid ETEC/STEC strains. These variants, increasingly documented in SDRS since 2021, present new challenges in field diagnosis and require close monitoring. On a herd level, veterinarians can use SDRS data to identify regional or age-specific trends, allowing them to proactively adapt herd health plans to shifting disease pressures.
Economic implications are also significant. Targeting interventions—such as adjusting vaccine selection in response to shifts in fimbriae types like the growing prevalence of F18 over the declining K88 (F4)—can reduce unnecessary costs while improving effectiveness. For epidemiologists and researchers, the new system facilitates clearer distinctions between pathogenic and non-pathogenic sample findings, thereby supporting future investigations into population-level trends.
Growing System Capacity for the Future
Dr. Trevisan stressed the broader implications of this report stating, “The addition of E. coli demonstrates how the SDRS can evolve with industry needs. It also positions the system for future incorporation of other bacterial pathogens. Just as importantly, this module serves as proof of concept that cross-laboratory collaboration coupled with targeted data curation can yield practical, accessible tools for the field.”
Future enhancements are being discussed for consideration. Stakeholders have expressed interest in integrating bacterial culture results and antimicrobial susceptibility profiles into the reporting system. Such additions would create a more holistic view of E. coli, potentially connecting molecular detection trends with real-world treatment outcomes. For producers, this would translate into even faster access to actionable intelligence that drives profitability and herd well-being.
The expansion of the SHIC Domestic Swine Disease Monitoring Reports to include E. coli genotyping and virotyping data provides valuable insights into the trends and geographic distribution of this pathogen. This information can be used to identify regional trends in virulence genes, inform disease control strategies, and reduce the production impact of E. coli on US swine.
SDRS
SHIC-funded Domestic Disease Surveillance Program: A collaborative project among multiple VDLs, with the goal to aggregate swine diagnostic data and report in an intuitive format (web dashboards and monthly PDF report), describing dynamics of pathogen detection by PCR-based assays over time, specimen, age group, and geographical area. Data is from the Iowa State University VDL, South Dakota State University ADRDL, University of Minnesota VDL, Kansas State University VDL, Ohio Animal Disease and Diagnostic Laboratory, and Purdue ADDL. For PRRSV ORF5 Lineages, RFLP, and variant classification data are also available from all participant d-labs. Specifically for syndromic dashboards, the results are from Iowa State University VDL.
SHIC
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments. As a conduit of information and research, SHIC encourages sharing of its publications and research. Forward, reprint, and quote SHIC material freely. For more information, visit http://www.swinehealth.org or contact Dr. Megan Niederwerder at [email protected] or Dr. Lisa Becton at [email protected].
In May 2025, the World Organisation for Animal Health (WOAH) adopted their first international standard for African swine fever (ASF) vaccines. With this recognition, the global swine health community has formally acknowledged the role that ASF vaccines may play in controlling and mitigating the disease. This is a major step forward, as WOAH standards are the basis for regulations recognized by the World Trade Organization (WTO) for international trade in animals and animal products. These standards directly shape countries’ import and export regulations. The Swine Health Information Center is pleased to share this update provided by Drs. Andres Perez and Rachel Schambow from the University of Minnesota College of Veterinary Medicine, Center for Animal Health and Food Safety.
Following the adoption of the standard, several countries asked WOAH to provide guidance for evaluating ASF vaccines in the field. In July 2025, WOAH convened a panel of experts from different regions and specialties to develop a set of guidelines. Dr. Perez served as a member of this WOAH ad hoc Group on ASF Vaccines: Field Evaluation and Post-Vaccination Monitoring. The first version of the guidelines developed by the panel has recently been released. Key points from these documents and relevant scientific publications include:
The science around ASF vaccines continues to evolve rapidly. When these guidelines were drafted, only Vietnam and the Philippines had implemented field use of ASF vaccines, and only in pigs older than 10 weeks destined for slaughter. Since both countries are net pork importers, there is little information yet on how international markets might respond to vaccine use. In this ever-changing space, this new standard and evaluation guidelines provide a framework for countries considering the use of ASF vaccines. Producers and veterinarians should stay engaged to understand how ongoing changes in ASF vaccine protocols and evaluation may impact them.

This month’s Domestic Swine Disease Monitoring Report shows that PRRSV case positivity in wean-to-market pigs has climbed to 41.46%, representing an increase of 6.28% compared to the same period last year. This emphasizes the need for stronger monitoring, biocontainment, and biosecurity practices in production sites. A bonus page highlights that PRRSV lineage 1C.5 continues to dominate, with 3,347 detections in 2025 already surpassing the total from 2024. Iowa alone accounts for 1,182 PRRSV 1C.5 cases. Illinois reported its first detection of PRRSV 1C.5.33. Pennsylvania had its first PRRSV 1C.2 detection in 2025; previous detections occurred in 2023 and 2024. Co-detection of IAV and PRRSV positive cases rose from 6% in July to over 10% in September, with IAV case positivity experiencing a substantial increase, reaching 35.66% in the wean-to-market category. In the podcast, Dr. Daniel Boykin, senior veterinarian at Smithfield Foods, discusses rapid response and biocontainment for emerging PRRSV strains, tips on preparedness for biosecurity practices, and next steps in regional control of PEDV and M. hyopneumoniae.

In the October Global Disease Monitoring Report, information on African swine fever activity in the Island of Hispaniola, the Baltics, and Vietnam is detailed. Authorities in the Dominican Republic reported over 390 ASF outbreaks in 2025 to date. In Europe, Estonia and Latvia have reported the largest ASF outbreaks to date, with a total of more than 50,000 pigs affected. In Vietnam, over 970 ASF outbreaks had been reported across 28 provinces by July 2025, affecting more than 100,000 pigs, mostly on smallholder farms with low biosecurity. Vietnam outbreaks include ASF virus of both genotype II and recombinant genotype I+II strains. A special report shares information on an outbreak of Brucella suis biovar 2 in Denmark, the first in 25 years. The pathogen was found in a free-range pig herd in Herning on August 22, 2025, following confirmation by the French Agency for Food Safety and the Environment (ANSES) reference laboratory. The affected herd includes approximately 3,850 pigs, with reproductive losses but no mortalities reported.
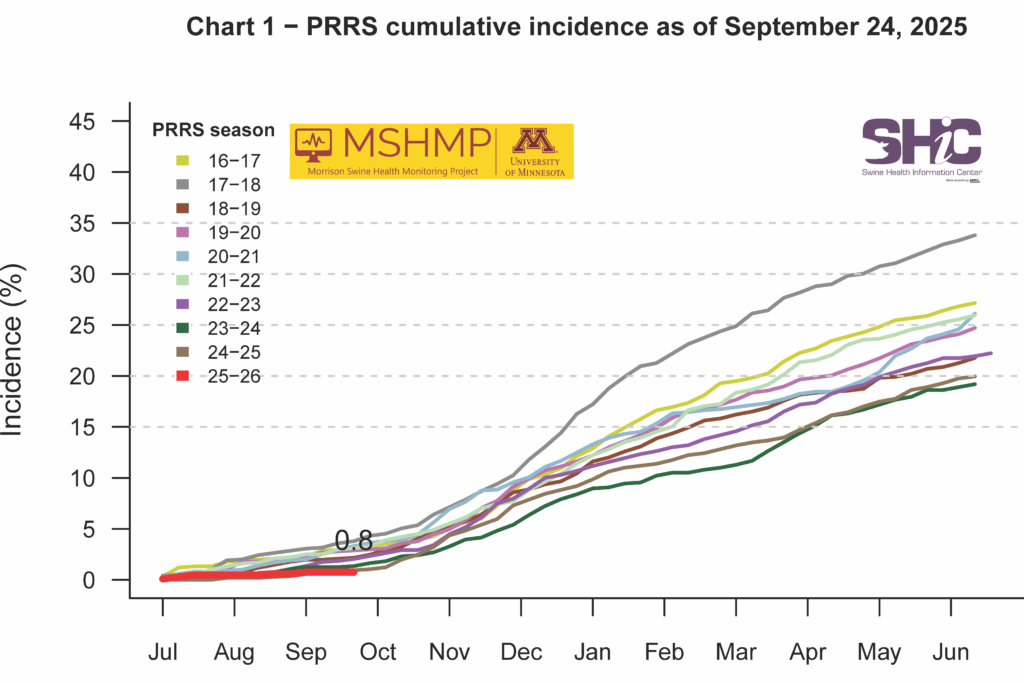
PRRS Cumulative Incidence for MSHMP
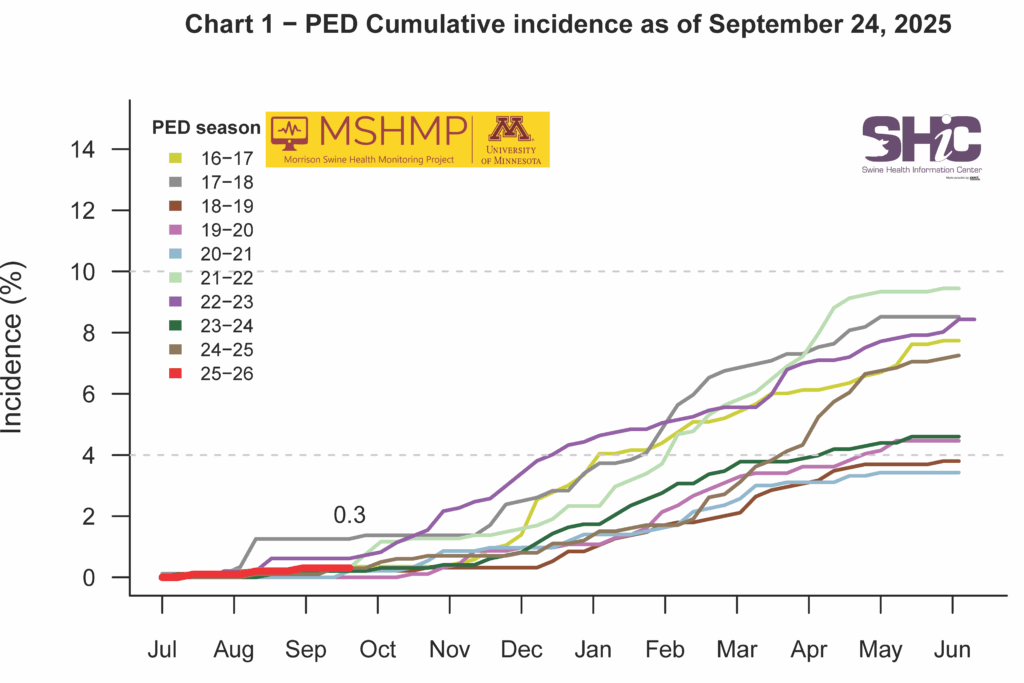
PEDV Cumulative Incidence for MSHMP
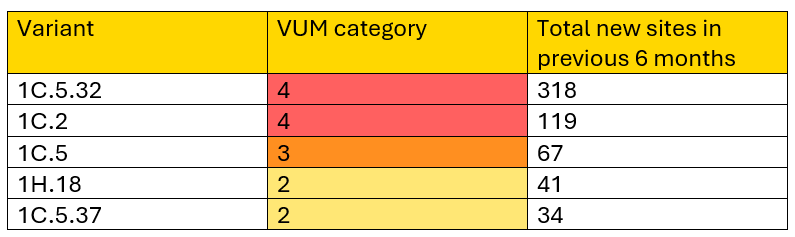
Five PRRSV variants were classified as Variants Under Monitoring (VUMs) category 2 or higher and are described in this month’s report: Variants 1C.5.32 (VUM Category 4), 1C.2 (VUM Category 4), 1C.5 (VUM Category 3), 1H.18 (VUM Category 2), and 1C.5.37 (VUM Category 2). Variant 1C.5.33 was demoted from VUM Category 2 to VUM Category 1 and a new variant-specific situation report was created. No other reports were generated this month, as the status of all other variants remained unchanged. Previous reports for all variants ever classified as VUM Category 2 or higher remain available.