
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments.

A collaborative team of investigators from North Carolina State University, USDA Agricultural Research Service, Iowa State University, and University of California Santa Cruz investigated if piglets infected with porcine astrovirus 4 exhibited reproducible lesions in the respiratory tract. Funded by SHIC, the study showed infected piglets shed the virus in nasal secretions, exhibited tracheitis and bronchitis with virus detected in tissues, and developed a productive immune response to infection. Principal investigator Dr. Michael Rahe, North Carolina State University, discusses results, which are the first to confirm that PoAstV4 can be a primary cause of epitheliotropic viral infection in the respiratory tract of piglets, with SHIC Executive Director Dr. Megan Niederwerder and host Barb Determan.
The Swine Health Information Center, in collaboration with the Foundation for Food & Agriculture Research and the Pork Checkoff, has recently funded 10 projects addressing research priorities and topics within its H5N1 Risk to Swine Research Program. Goals of the program are to enhance prevention, preparedness, mitigation, and response capabilities for H5N1 influenza in the US swine herd. The priority areas addressed through the funded projects include vaccine development and cross-protection, clinical presentation of pigs across different production phases, potential for mammary transmission, diagnostic surveillance, introduction and transmission risks, and biosecurity practices. The 10 new projects were initiated in summer 2025 and are 12 to 18 months in duration. Research outcomes from the funded projects will be shared with producers and veterinarians as soon as they become available.
The SHIC/FFAR/NPB H5N1 Risk to Swine Research Program request for proposals was announced on November 6, 2024, and received 51 proposals from 35 different institutions across six countries by the submission deadline of December 31, 2024. Proposals underwent a competitive review process across the first several months of 2025 by subject matter experts on influenza and the swine industry. Funding awarded across the 10 new projects totals $2.1 million of the $4 million total available for this program. Funding timely research is an essential component of SHIC providing project outcomes that drive action for emerging disease prevention, preparedness, mitigation, and response for the US swine industry.
SHIC/FFAR/NPB H5N1 Risk to Swine Research Program projects funded and initiated in response to the RFP include:
Vaccine Development and Cross-Protection
 Development of a vaccine against highly pathogenic avian influenza viruses for use in pigsDevelopment of a vaccine against highly pathogenic avian influenza viruses for use in pigs
Development of a vaccine against highly pathogenic avian influenza viruses for use in pigsDevelopment of a vaccine against highly pathogenic avian influenza viruses for use in pigs Evaluation of cross-protective N1 swine antibodies against HPAI H5N1 clade 2.3.4.4b virus
Evaluation of cross-protective N1 swine antibodies against HPAI H5N1 clade 2.3.4.4b virus  Role of Prior Immunity to endemic swine viruses on H5N1 infection in pigs
Role of Prior Immunity to endemic swine viruses on H5N1 infection in pigs Preparing the US Swine Industry for HPAI H5N1: Quantifying and comparing H5N1 vs H1N1 spreading, shedding, and detection in groups with or without immunity to H1N1.
Preparing the US Swine Industry for HPAI H5N1: Quantifying and comparing H5N1 vs H1N1 spreading, shedding, and detection in groups with or without immunity to H1N1. Pathogenesis and transmission studies in piglets and sows infected with different genotypes of 2.3.4.4b clade H5Nx viruses
Pathogenesis and transmission studies in piglets and sows infected with different genotypes of 2.3.4.4b clade H5Nx viruses  Evaluating H5N1 risk to swine: mammary transmission and clinical presentation in lactating sows
Evaluating H5N1 risk to swine: mammary transmission and clinical presentation in lactating sows Investigation of susceptibility of porcine mammary gland to highly pathogenic avian influenza (H5N1) virus infection and transmission risk of virus from sows to nursing piglets
Investigation of susceptibility of porcine mammary gland to highly pathogenic avian influenza (H5N1) virus infection and transmission risk of virus from sows to nursing pigletsDiagnostic Surveillance
 An efficient and rapid automation workflow to detect influenza subtypes using RT-qPCR assay via 96-well Veriflex heat blocks on QuantStudio 7 Pro
An efficient and rapid automation workflow to detect influenza subtypes using RT-qPCR assay via 96-well Veriflex heat blocks on QuantStudio 7 ProIntroduction and Transmission Risks/Biosecurity Practices
 Pathogenesis and interspecies transmission of H5N1 influenza virus in swine
Pathogenesis and interspecies transmission of H5N1 influenza virus in swine Enhancing Biosecurity in Swine Operations: Investigating Wildlife Interactions on Swine Farms
Enhancing Biosecurity in Swine Operations: Investigating Wildlife Interactions on Swine FarmsCritical research investments are necessary to understand and prevent H5N1 incursion on swine farms, ensure rapid detection of H5N1 if introduced, protect animal and caretaker health, inform pork industry stakeholder response, mitigate production losses on farm, identify effective control measures, and develop clear messaging to consumers on the safety of pork. Outcomes from the funded projects will provide critical information that producers, veterinarians, and industry stakeholders can use to better prevent incursion and develop preparedness plans if H5N1 is identified in US commercial swine herds.

The US swine industry has proactively united to combat the potential threat of H5N1 influenza, a highly pathogenic avian influenza (HPAI) that has impacted poultry flocks and dairy herds, humans, and a few pigs on an isolated Oregon hobby farm. Since March 2024, a dedicated working group, comprised of representatives from the American Association of Swine Veterinarians (AASV), National Pork Board (NPB), National Pork Producers Council (NPPC), and Swine Health Information Center (SHIC), has been working continuously. Their mission: to prevent and prepare for a potential H5N1 outbreak response in commercial US swine herds.
Collaboration: A Unified Front Against H5N1
The emergence of H5N1 in dairy cattle in March 2024 was an eye-opening occurrence, prompting the immediate formation of the swine industry working group. Dr. Heather Fowler, director of producer and public health, NPB, emphasizes the need for multi-species, interdisciplinary collaboration to adequately prepare for and respond to an H5N1 outbreak. Dr. Marisa Rotolo, director of swine health, NPB, echoes this, highlighting how the outbreak has prompted livestock commodity groups to strengthen relationships and share knowledge across the barnyard.
This unified approach ensures a more efficient and effective response. As Dr. Fowler states, “By collaborating we are not only sharing the load but also making sure the right people are involved in the conversation at the right time.” This synergy leverages the unique strengths and expertise of each organization, ultimately benefiting producers and veterinarians. AASV, for instance, has leveraged its committees, comprised of global experts in influenza viruses, to review and provide feedback on the draft response plan, ensuring it is as realistic and useful as possible.
Single H5N1 Diagnosis in U.S. Backyard Pig
In October 2024, the US swine industry learned of the first detection of H5N1 influenza in a pig on a small backyard mixed-species farm in Oregon. NPPC and SHIC immediately shared this detection with pork industry stakeholders. SHIC prepared and deployed both a timely eblast and monthly newsletter article. While the detection on the Oregon farm was isolated and the crossover to swine limited, it reinforced efforts by SHIC, NPB, NPPC, and AASV to remain diligent in ongoing endeavors to monitor H5N1 spread and learn more about its risks to commercial swine.
“Ensuring timely and valuable communications across all stakeholder audiences is part of the SHIC mission to minimize the impact of emerging diseases,” said Dr. Megan Niederwerder, executive director of SHIC. “Confirmation of H5N1 in a backyard pig by USDA raised questions regarding this emerging threat and coordinating communications to inform veterinarians and producers was critical.”
Proactive Measures and Key Learnings
Stakeholders recognized the importance of being at the forefront of planning. Dr. Abbey Canon, director of public health and communications, AASV, notes that swine veterinarians and producers understood it’s best to be at the table and be part of the planning process, a foresight commended by USDA and state animal health officials.
A critical realization for the working group has been the complex nature of an H5N1 response, extending beyond the swine industry itself. Dr. Anna Forseth, director of animal health, NPPC, points out that the process has been far more than just what is best for the swine industry alone, considering the zoonotic potential, susceptibility in other species, and the foreign animal disease classification for poultry.
Early on, SHIC started gathering broad input with industry partners from veterinarians, pork producers, and state/federal animal health officials on potential gaps in knowledge and research priorities for H5N1 in commercial swine operations. “We wanted to understand what research questions would generate the highest value data and information for US pork producers,” noted Dr. Niederwerder.
Organizational Contributions and Achievements
Each participating organization has played a vital role in the working group’s efforts:
 National Pork Board: NPB has supported the development of an H5N1 response guide and facilitating regular calls across industry groups to ensure timely updates. They have created a dedicated H5N1 landing page on PorkCheckoff.org and disseminated existing resources like the Secure Pork Supply plan. Crucially, NPB has partnered with SHIC and the Foundation for Food & Agriculture Research (FFAR) to fund a significant research initiative focused on H5N1, aiming to fill critical knowledge gaps.
National Pork Board: NPB has supported the development of an H5N1 response guide and facilitating regular calls across industry groups to ensure timely updates. They have created a dedicated H5N1 landing page on PorkCheckoff.org and disseminated existing resources like the Secure Pork Supply plan. Crucially, NPB has partnered with SHIC and the Foundation for Food & Agriculture Research (FFAR) to fund a significant research initiative focused on H5N1, aiming to fill critical knowledge gaps. National Pork Producers Council: NPPC took the lead in drafting the comprehensive guidance response plan, presenting it to USDA in January 2025. This plan underwent rigorous review and feedback from a wide array of stakeholders, including AASV’s influenza committee and Board, NPB and NPPC Boards, and public health professionals, ensuring broad acceptance and practical applicability.
National Pork Producers Council: NPPC took the lead in drafting the comprehensive guidance response plan, presenting it to USDA in January 2025. This plan underwent rigorous review and feedback from a wide array of stakeholders, including AASV’s influenza committee and Board, NPB and NPPC Boards, and public health professionals, ensuring broad acceptance and practical applicability. Swine Health Information Center: SHIC has been at the forefront of timely information dissemination and research prioritization. Upon the first detection of H5N1 in a backyard pig in Oregon in October 2024, SHIC and co-sponsor AASV immediately alerted stakeholders and organized a webinar, “H5N1 Influenza Risk to US Swine,” to provide the latest information and address concerns. SHIC also spearheaded the H5N1 Risk to Swine Research Program, a $4 million initiative in partnership with the Foundation for Food & Agriculture Research and the Pork Checkoff, to fund targeted research addressing crucial knowledge gaps. The overwhelming response to their Request for Proposals, with 51 submissions from 35 institutions across six countries, underscores the industry’s commitment to scientific understanding. SHIC has funded 10 projects from the submissions with work scheduled to begin in summer 2025.
Swine Health Information Center: SHIC has been at the forefront of timely information dissemination and research prioritization. Upon the first detection of H5N1 in a backyard pig in Oregon in October 2024, SHIC and co-sponsor AASV immediately alerted stakeholders and organized a webinar, “H5N1 Influenza Risk to US Swine,” to provide the latest information and address concerns. SHIC also spearheaded the H5N1 Risk to Swine Research Program, a $4 million initiative in partnership with the Foundation for Food & Agriculture Research and the Pork Checkoff, to fund targeted research addressing crucial knowledge gaps. The overwhelming response to their Request for Proposals, with 51 submissions from 35 institutions across six countries, underscores the industry’s commitment to scientific understanding. SHIC has funded 10 projects from the submissions with work scheduled to begin in summer 2025. American Association of Swine Veterinarians: AASV’s dedicated committees, comprised of leading experts, have provided invaluable scientific and practical input into the response plan. They advocate for continued participation in existing influenza A virus surveillance programs, emphasizing their role in early detection and rapid response to emerging threats. AASV also actively promotes preparedness programs like AgView® and the Secure Pork Supply Plan as well as co-sponsoring timely webinars on H5N1.
American Association of Swine Veterinarians: AASV’s dedicated committees, comprised of leading experts, have provided invaluable scientific and practical input into the response plan. They advocate for continued participation in existing influenza A virus surveillance programs, emphasizing their role in early detection and rapid response to emerging threats. AASV also actively promotes preparedness programs like AgView® and the Secure Pork Supply Plan as well as co-sponsoring timely webinars on H5N1.Lessons from Other Livestock Industries
The swine industry has closely observed the H5N1 responses in the poultry and dairy sectors, gleaning valuable lessons to inform its own strategy.
From the Poultry Industry
The poultry industry’s experience with H5N1, where it is classified as a foreign animal disease, has provided key insights:
 Significant Investment: The substantial USDA investment ($1 billion) in the poultry response highlights the financial commitment required for combating widespread outbreaks, particularly concerning biosecurity, indemnity, and vaccine development.
Significant Investment: The substantial USDA investment ($1 billion) in the poultry response highlights the financial commitment required for combating widespread outbreaks, particularly concerning biosecurity, indemnity, and vaccine development. Program Value: The National Poultry Improvement Plan (NPIP) demonstrates the value of established programs in maintaining exports and controlling disease spread, offering a potential model for the swine industry’s U. S. Swine Health Improvement Plan (U.S. SHIP).
Program Value: The National Poultry Improvement Plan (NPIP) demonstrates the value of established programs in maintaining exports and controlling disease spread, offering a potential model for the swine industry’s U. S. Swine Health Improvement Plan (U.S. SHIP). Biosecurity and Indemnity: The poultry experience has raised questions about the level of biosecurity that may be required for swine farms and how current industry programs could integrate with indemnity considerations.
Biosecurity and Indemnity: The poultry experience has raised questions about the level of biosecurity that may be required for swine farms and how current industry programs could integrate with indemnity considerations. Vaccine Discussions: The ongoing discussions around vaccine development for poultry are closely monitored, as understanding an industry need, development timelines, and potential trade barriers will be crucial for any future swine vaccine strategy.
Vaccine Discussions: The ongoing discussions around vaccine development for poultry are closely monitored, as understanding an industry need, development timelines, and potential trade barriers will be crucial for any future swine vaccine strategy.From the Dairy Industry
The dairy industry’s H5N1 experience, where the virus is regulated differently, has offered unique lessons:
 Movement Restrictions: While not universal, the implementation of quarantines and movement restrictions for certain dairy cattle types has highlighted potential challenges for the swine industry, which relies heavily on interstate movements.
Movement Restrictions: While not universal, the implementation of quarantines and movement restrictions for certain dairy cattle types has highlighted potential challenges for the swine industry, which relies heavily on interstate movements. Patchwork State Requirements: The emergence of varied state-specific import requirements in the dairy sector serves as a warning for the swine industry, emphasizing the need for coordinated, national guidelines.
Patchwork State Requirements: The emergence of varied state-specific import requirements in the dairy sector serves as a warning for the swine industry, emphasizing the need for coordinated, national guidelines. Exhibition Sector Focus: The dairy experience has underscored the vulnerability of the exhibition sector due to frequent commingling and human interaction, leading to a specific focus on this area within the swine guidance response plan.
Exhibition Sector Focus: The dairy experience has underscored the vulnerability of the exhibition sector due to frequent commingling and human interaction, leading to a specific focus on this area within the swine guidance response plan. Worker Safety and Surveillance: The enhanced focus on worker safety and surveillance in dairy operations reinforces the importance of similar measures in swine production.
Worker Safety and Surveillance: The enhanced focus on worker safety and surveillance in dairy operations reinforces the importance of similar measures in swine production. Pork Safety Studies: The need for milk and beef safety studies in dairy suggests that similar pork safety studies will be crucial if H5N1 were to enter the swine herd.
Pork Safety Studies: The need for milk and beef safety studies in dairy suggests that similar pork safety studies will be crucial if H5N1 were to enter the swine herd.Recommendations for Pork Producers and Veterinarians
The working group’s efforts translate into actionable recommendations for pork producers and veterinary practitioners:
 Stay Informed: Dr. Rotolo strongly encourages producers and veterinarians to stay up to date with H5N1 developments.
Stay Informed: Dr. Rotolo strongly encourages producers and veterinarians to stay up to date with H5N1 developments. Enhance Biosecurity: This is paramount. Drs. Forseth and Rotolo shared recommendations include implementing practices aimed at preventing interaction between pigs and other species, paying particular attention to potential introduction pathways such as wild birds, contaminated feed or water, raw milk feeding, or shared labor/equipment with poultry or dairy facilities. Further biosecurity resources, including recommendations for show pig participants, are available at porkcheckoff.org.
Enhance Biosecurity: This is paramount. Drs. Forseth and Rotolo shared recommendations include implementing practices aimed at preventing interaction between pigs and other species, paying particular attention to potential introduction pathways such as wild birds, contaminated feed or water, raw milk feeding, or shared labor/equipment with poultry or dairy facilities. Further biosecurity resources, including recommendations for show pig participants, are available at porkcheckoff.org. Participate in Surveillance: AASV recommends active participation in IAV surveillance programs to monitor virus evolution and epidemiology, enabling early detection and rapid response to emerging threats.
Participate in Surveillance: AASV recommends active participation in IAV surveillance programs to monitor virus evolution and epidemiology, enabling early detection and rapid response to emerging threats. Engage in Preparedness Programs: Producers are urged to sign up for AgView®, participate in the Secure Pork Supply Plan, and explore the Certified Swine Sample Collector Program. These initiatives provide crucial tools for disease preparedness and response.
Engage in Preparedness Programs: Producers are urged to sign up for AgView®, participate in the Secure Pork Supply Plan, and explore the Certified Swine Sample Collector Program. These initiatives provide crucial tools for disease preparedness and response. Prioritize Worker Health: Consistent recommendations include not coming to work when sick and getting annual influenza vaccinations, protecting both human and animal health.
Prioritize Worker Health: Consistent recommendations include not coming to work when sick and getting annual influenza vaccinations, protecting both human and animal health.While H5N1 preparedness and response planning share similarities with efforts surrounding foreign animal diseases like African swine fever (ASF), foot-and-mouth disease (FMD), and classical swine fever (CSF), there are key distinctions.
Dr. Rotolo highlights that the ultimate response to H5N1 detection in any livestock species rests with federal and state partners. While H5N1 is a foreign animal disease for poultry and typically results in depopulation, the approach for dairy herds has differed, with no depopulation. This suggests that a similar approach might be taken for swine, unlike the typical eradication strategy for ASF, CSF, and FMD.
Dr. Forseth notes that common parallels include potential quarantine, movement restrictions, establishment of surveillance zones, surveillance for epi-linked premises, and potential trade implications. However, the emerging and somewhat unknown nature of the virus in swine underscores the importance of the working group’s proactive planning to define the precise response.
The Enduring Commitment: Protecting People, Pigs, and the Planet
The sustained interest in the H5N1 swine working group, even after a year of continuous collaboration, is a testament to the shared passion and common goal of its members. Dr. Fowler said it’s about, “Shared passion, shared goal, and good people.” Dr. Rotolo adds that the inherent susceptibility of pigs to influenza A, combined with the ongoing transmission of novel strains, makes it as important as ever for the swine industry to remain vigilant and ready to adapt to changes in disease threats. The constant domestic and international threat of H5N1, particularly with wild birds as uncontrollable vectors, keeps the mission at the forefront.
The Influence of Zoonotic Potential
The zoonotic nature of H5N1 significantly influences the working group’s goals and information sharing. It necessitates a comprehensive One Health approach, integrating animal health, human health, and environmental health. Dr. Fowler emphasizes the importance of protecting people, pigs, and the planet in the face of a zoonotic pathogen with multiple animal hosts. This aspect fosters crucial collaboration with partners in other species and industry groups, as well as with human health colleagues.
“The zoonotic component is very important. This virus is challenging because of its ability to change. A change may make it more difficult to control in animals or more of a threat to people. A large component of the response plan focused on worker safety and public health,” Dr. Forseth remarks.
The swine industry’s long-standing, successful relationship with public health partners has been invaluable, ensuring that worker safety and public health are central to the response plan. AASV actively supports recommendations for people working with swine to take all available precautions, including vaccination, biosecurity, and personal protection, to prevent bidirectional influenza transmission.
“The swine industry is lucky to have a long, successful working relationship with our partners in public health, especially veterinary public health and zoonotic diseases,” Dr. Canon states. “Labor is a top issue in pork production, and we want to protect our people and make sure they feel safe coming to work. Public health professionals were involved in drafting this response from the beginning, and animal and human health goals are aligned.”
Looking Ahead: Continued Vigilance, Research, and Refinement
The working group remains committed to its mission. NPB will continue to engage in H5N1 learnings, particularly concerning research updates on the epidemiology of the virus, to better inform and prepare producers. NPPC plans to continue conversations with USDA and further refine the guidance response plan. AASV is focused on disseminating more details about the plan to swine veterinarians and producers, having already initiated these conversations at events like the World Pork Expo in June 2025.
SHIC will shepherd H5N1 research projects; 10 projects were selected for funding in 2025 with awards totaling $2.1 million. Projects were initiated in summer of 2025. “Awarded projects are addressing diverse H5N1 research questions, such as the clinical signs in pigs across different production phases, the potential for mammary transmission in sows, if endemic influenza provides cross-protective immunity, vaccine efficacy, diagnostic test sensitivity, and transmission risks through wildlife exposure,” commented Dr. Niederwerder.
The overarching message for pork producers, veterinarians, and industry stakeholders is clear: the swine industry is proactively addressing the H5N1 threat. Efforts are underway to protect the US swine herd, ensuring the safety of workers, and safeguarding the industry’s future. The working group welcomes feedback as it continues to work on behalf of the industry to protect people, pigs, and the planet.

A recent study funded by the Swine Health Information Center underscores the significant threat Japanese encephalitis virus (JEV) poses to the global swine industry. The study, led by Dr. Natalia Cernicchiaro at Kansas State University, in collaboration with USDA Agricultural Research Service scientists at the National Bio and Agro-Defense Facility, included a systematic literature review and meta-analysis on JEV vector and host competence. Key findings from experimental studies reveal that at least nine additional mosquito species can be potential vectors for JEV, nearly half of the mosquitoes exposed to JEV became infected, and that one in four infected mosquitoes can transmit JEV to hosts. Culex species of mosquitos may pose the greatest risk to humans and animals, including swine. Findings emphasize the need for robust surveillance and integrated mosquito management strategies for US pork producers. The full report published by Parasites & Vectors can be accessed here.
Japanese encephalitis is an emerging zoonotic disease transmitted by JEV-infected mosquitos. It is considered a significant human and animal health threat. The virus is primarily maintained in its natural life cycle between mosquitoes and waterbirds, while occasionally spilling over into swine, horses, and humans. JEV infections in swine result in reproductive disease outcomes on sow farms, such as stillbirths, mummified fetuses, and abortions. While JEV has not been detected in the US, its recent spread globally, coupled with the presence of competent mosquito vectors and susceptible hosts in the US, elevates the risk of incursion.
Considering the recent expansion of JEV into mainland Australia and increased global research activity, this systematic review summarizes new experimental data on mosquito vector competence published between 2016 and 2023, building on a 2018 review previously published by the investigators. All reports included in the review were peer-reviewed literature and screened for relevance to vector competence, with a focus on JEV infection, dissemination, and transmission rates as the primary outcomes of interest. The study population was limited to mosquito vectors, and only experimental studies were eligible.
The updated meta-analysis revealed several crucial points for US pork producers and stakeholders to consider. First, the study found an overall JEV infection rate of 45.4% across 51 unique mosquito species, meaning that nearly half of the mosquitoes exposed to JEV in experimental settings became infected. This highlights the significant potential for virus amplification in mosquito populations around livestock, including pig farms.
The transmission rate across 30 experimentally tested mosquito species was 22.7%, indicating that roughly one in four of the tested infected mosquito species are capable of transmitting JEV to susceptible hosts, such as animals and humans. This underscores the importance of mosquito control measures in commercial swine operations to reduce the risk of an outbreak if the virus ever enters the country.
While vector competence for JEV varied by mosquito species, Culex mosquitoes exhibited the highest infection (51.9%) and transmission (27.8%) rates among all the mosquito species tested experimentally. These species are usually common in human and livestock settings, making them high-priority targets for surveillance and control.
The review identified an additional nine to 12 new mosquito species with demonstrated competence for JEV, expanding the list of potential mosquito vectors beyond those identified in the previous review. This continuous identification of new competent species emphasizes the dynamic nature of JEV epidemiology and the importance of ongoing systematic reviews to maintain up-to-date information essential for effective surveillance programs.
Data on which mosquito species are most likely to carry and spread JEV can help focus surveillance and control efforts on the species that pose the greatest risk. Some mosquito species can serve as early warning signals of JEV activity and may play key roles as primary or secondary vectors. However, infection rates alone are not enough, factors such as mosquito abundance, feeding habits, lifespan, and proximity to humans and animals are also important.
Understanding how efficiently mosquitoes spread JEV is essential for assessing the risk of the virus appearing in new areas. Identifying the species most capable of transmitting JEV allows for targeted control strategies to reduce infections and prevent the spread of the virus to vulnerable regions.
In their conclusion, the researchers state, “This review provides updated data not only on newly reported species showing competence for JEV but also on the level of competence observed in previously identified species. These findings offer valuable insights that go beyond what individual studies can provide, helping to synthesize the evidence base in a way that is accessible and actionable for policymakers, public health authorities, and disease modelers. Ultimately, the goal is to support evidence-based decision-making with the most current and comprehensive data available.”
If referencing this article’s findings, please cite the original study by Edache et al., 2025 published in Parasites & Vectors.

Senecavirus A is an endemic pathogen that remains an ongoing concern to the US swine industry. Its clinical presentation is characterized by vesicular lesions on the snout and feet, closely mimicking those caused by economically devastating foreign animal diseases such as foot-and-mouth disease. A recent publication, led by Drs. Mariana Kikuti, Cesar Corzo, and the Morrison Swine Health Monitoring Project team at the University of Minnesota, examines how frequently new SVA outbreaks occur in breeding herds. This work helps to understand virus spread, as well as when and where it is most detected, and estimates the cumulative incidence of SVA in the US to quantify disease burden. Published in the journal Animals (2025,15,1650), results provide epidemiologic insights into SVA across US breeding herds from January 2015 to December 2024 through analysis of data gathered from the SHIC-funded MSHMP.
As an endemic pathogen, SVA impacts animal health through causing vesicles as well as lameness and lethargy. Further, SVA is clinically indistinguishable from FMD, making investigations for every suspected case necessary to rule out a foreign animal disease and placing a significant burden on state and federal animal health agencies. A major SVA outbreak in 2015 raised concerns across the US swine industry. However, since then, there has been a gap in comprehensive research on how often SVA occurs across US pig farms.
Data included in this study is comprised of SVA PCR results originating from production systems participating in MSHMP as well as the type of specimen submitted and the official Premises Identification Number. Currently, the sow population participating in MSHMP represents approximately 60% of the total US breeding herd. As MSHMP involves a dynamic cohort, the number of breeding herds monitored throughout the study period varied, ranging between 1063 and 1183 sites per calendar year. Through the laboratory surveillance, a total of 36,400 SVA PCR submissions were provided by the University of Minnesota and Iowa State University VDLs from January 2015 to December 2024. This robust dataset allowed for the assessment of SVA incidence, identification of temporal fluctuations, and characterization of regional patterns.
Despite its clinical significance, the cumulative incidence of SVA in US breeding herds remained low, generally less than 2.5% per year across the 10-year study period. This suggests that while SVA continues to circulate, it affects a relatively small proportion of breeding herds annually. For sites experiencing more than one SVA outbreak, the median time interval between outbreaks was approximately 402 days, highlighting the potential for re-introductions and/or persistent circulation within herds.
A notable temporal pattern was observed, with peak SVA incidence occurring during the third and fourth quarters of the calendar year (July to December). This suggests seasonality influences disease transmission dynamics. A compilation of reports from multiple VDLs further supports this observation, with a consistent increase in the frequency of SVA cases during summer months. This seasonality requires further investigation, particularly given the limited understanding of between-farm transmission risk factors for SVA.
Regional patterns were also identified, with SVA outbreaks more frequently reported in the Midwest region of the US. Though this finding is confounded with the high density of swine production in the Midwest, efforts to better assess disease distribution are still needed. The temporal and regional patterns suggest seasonal fluctuations and a regional disease burden, emphasizing the need for continued surveillance to better understand SVA dynamics across the country.
As discussed in the study, the potential role of factors like personnel and animal movement, dead animal management, fomites (e.g., trailers) and even potential vectors (e.g., flies) in SVA transmission reinforces the importance of stringent biosecurity protocols. Further, the study highlighted the ongoing gap in knowledge for SVA epidemiology in growing pig populations, which were not the primary focus of this study but are known to be affected.
Overall, this study provides valuable, data-driven insights into the current epidemiology of SVA, enabling veterinarians and producers to enhance prevention and control strategies. It underscores the ongoing need for robust and collaborative surveillance systems that integrate on-farm observations with laboratory diagnostics to provide a comprehensive picture of SVA dynamics. Specifically, the observed seasonality and regional concentration suggest opportunities for more targeted biosecurity enhancements and surveillance efforts, particularly in the Midwest during the latter half of the year.
While SVA’s overall incidence in US breeding herds remains low, continued awareness of SVA as a differential for vesicular lesions is paramount to promptly trigger an FAD investigation to rule-out trade-limiting diseases such as FMDV. This decade-long surveillance data from MSHMP, a project supported by SHIC, serves as a crucial resource for the US swine industry, informing strategies to safeguard animal health and ensure industry stability. Ultimately, this work underscores the importance of collaborative data sharing among producers, veterinarians, and academic institutions to improve the management of SVA and other swine diseases in the US.
Reference:
Kikuti, M.; Yue, X.; Melini, C.M.; Vadnais, S.; Corzo, C.A., Senecavirus A Incidence in U.S. Breeding Herds: A Decade of Surveillance Data, MDPI Animals (2025, 15,1650). https://doi.org/10.3390/ani15111650

This month’s Domestic Swine Disease Monitoring Report covers the reduction in PRRSV case positivity across all age categories, with overall positivity dropping below 20%. However, PRRSV lineage L1C.5 had several detections mostly concentrated in Iowa (175). In addition, after the first detection in North Carolina, the PRRSV lineage L1C.2 continues to be detected there and elsewhere in the US. Mycoplasma hyopneumoniae reached the lowest monthly case positivity in sow farms since 2012. In the ISU-VDL, confirmed tissue diagnosis of MHP had the lowest number of cases in the second quarter of 2025. The overall percentage of PCV3 positive submissions increased sharply in July, surpassing PCV2 overall case positivity by 18%. Most of the July PCV3 positive samples were processing fluids (310/475) followed by oral fluids (85/475). In the podcast, Maria Pieters, University of Minnesota Associate Professor, discussed the Mycoplasma hyopneumoniae elimination trends in the US, new diagnostic tools, success rates of elimination protocols, and particularities to address for each farm.

In the August Global Swine Disease Monitoring Report, read about foot-and-mouth disease in South Africa, where 27 new outbreaks were reported across five provinces as the triple serotype (SAT 1, 2 and 3) threat escalates. In Estonia, the first report of African swine fever since 2023 occurred, with multiple outbreaks in commercial farms confirmed and over 17,000 pigs culled. In Germany, ASF spread to a second district in North Rhine-Westphalia, raising concerns as the outbreak zone lies only 93 miles from Belgium and the Netherlands. Also, read about the two main clades of Nipah virus in South and Southeast Asia – Bangladesh clade (NiV-B) and Malaysia clade (NiV-M) – in a special section.
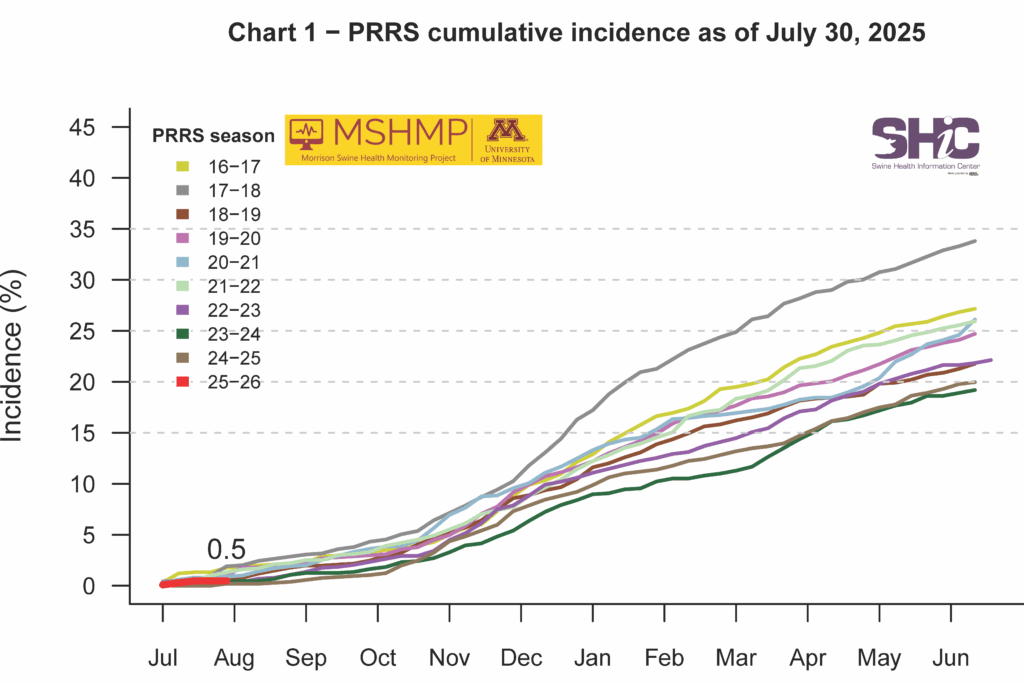
PRRS Cumulative Incidence for MSHMP Beginning July 1, 2009
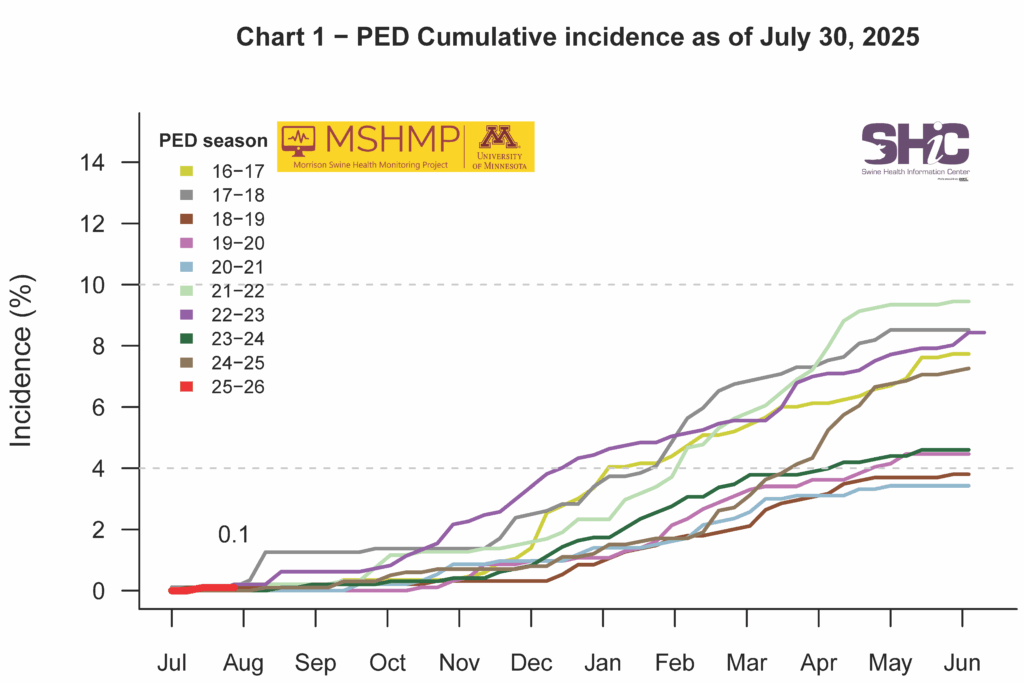
PEDV Cumulative Incidence for MSHMP Beginning May 1, 2013