

Veterinarians receiving diagnostic test results of porcine astrovirus 3, porcine sapelovirus, or porcine teschovirus may need more information to improve management of the CNS syndrome associated with these viruses. To address these challenges, the Swine Health Information Center (SHIC), in collaboration with the American Association of Swine Veterinarians (AASV), will hold a webinar titled Disease Management of Viral Myelitis for veterinary practitioners and pork producers on December 4, 2019, from 10:00 am to 11:30 am CST.
This webinar will include a brief overview of viral myelitis as well as current diagnostic tools. Diagnosticians Dr. Bailey Arruda, Iowa State University Veterinary Diagnostic Lab (VDL), and Dr. Matthew Sturos, University of Minnesota VDL, will talk about their experiences with cases and ways to approach management from the perspective of scientific design. Practitioners will present management approaches they have used when they receive these diagnoses. Click here for the working agenda for the Disease Management of Viral Myelitis webinar.
Registration for this SHIC/AASV webinar is required. Registered participants will receive information for webinar participation, which is limited, so please register soon. Click here for information on how to utilize the webinar platform, Zoom.
The webinar will be recorded and available for viewing on the both the SHIC and AASV websites following the event.

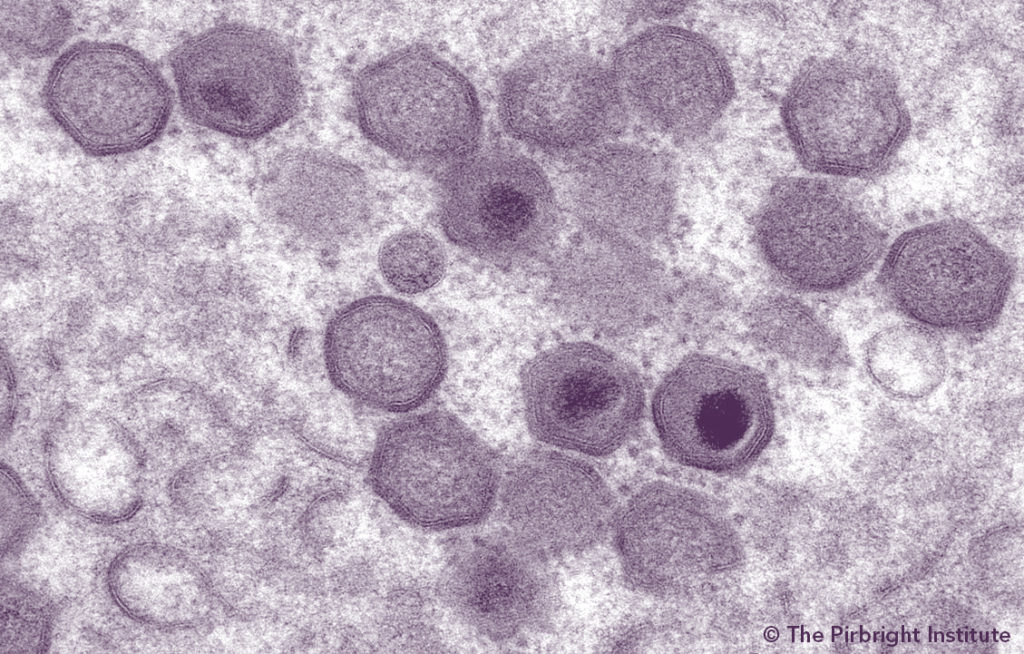
Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) was isolated from two recent cases of high sow and feeder pig mortality in US assembly yards. While this organism is a sporadic cause of disease in multiple animal species, it has rarely been associated with disease outbreaks in US swine. Work completed by the Iowa State University Veterinary Diagnostic Lab (ISU VDL) since the initial diagnosis, with support from the Swine Health Information Center (SHIC), provides more details on current concerns.
See the initial report on this occurrence of S. zooepidemicus here.
Based on review of past cases, ISU VDL learned the recent US strains are strikingly similar to the ATCC strain isolated from swine outbreak(s) of high mortality in China in the mid-1970s that reportedly involved the loss of more than 300,000 pigs (1, 2). These further characterization results suggest these recent case series for US assembly yards were related and caused by the same variant of S. zooepidemicus. Previous isolations of S. zooepidemicus from clinically ill pigs have been rather limited in the US. There is minimal information concerning the novelty of this particular variant of S. zooepidemicus, as compared to past findings in US swine.
Elevated swine mortalities in western Canadian assembly yards due to S. zooepidemicus were reported in the second quarter of 2019, per the Canadian West Swine Health Intelligence Network. More recently, an additional incident of elevated mortality and abortions caused by S. zooepidemicus in four related commercial sow farms located in Manitoba, Canada, occurred. The S. zooepidemicus isolate from the case series involving the four commercial sow farms in Manitoba was also reported to be genetically similar to the aforementioned ATCC strain of S. zooepidemicus associated with the high swine mortality event in Sichuan province in China in the 1970s(3).
These occurrences in the US and western Canada should spur increased pig farm biosecurity efforts, particularly related to transport and collection points.
SHIC will continue its efforts as further study is needed to better understand the relevance and prevalence of S. zooepidemicus in North American swine.
References
An updated Swine Health Information Center (SHIC) Diagnostic Assay Catalog has been posted and now includes Enzyme Linked Immunosorbent Assays (ELISAs) for detection of antibodies in serum and oral fluids against Hepatitis E, sapelovirus, teschovirus, swine influenza virus, porcine circovirus 3 (PCV3), and atypical porcine pestivirus in addition to 18 Polymerase Chain Reaction (PCR) Assays. The initial SHIC PCR Assay Catalog, published in 2018, contained 17 SHIC-funded tests. The catalog illustrates how far the pork industry has advanced in the ability to test for emerging diseases as well as how SHIC is fulfilling its mission of diagnostic preparedness and readiness for possible new or emerging production diseases.
The catalog provides diagnosticians with pertinent information about new and existing PCR and ELISA tests developed, including contact information of the experts for questions about availability and use. Additionally, the catalog summarizes the research behind the test development and covers technical background information including sample types and analytical and diagnostic sensitivity and specificity.
From evaluating risks via the SHIC Swine Disease Matrix and assessing current diagnostic needs to be able to quickly identify these pathogens, to funding the development of tests, SHIC has led the pork industry to an enhanced level of emerging disease readiness.
Recognizing limitations on producer resources may be a barrier, SHIC also offers Diagnostic Fee Support in cases of high morbidity/high mortality, where an etiology is either not identified or there is a strong supposition the identified pathogen is not the likely cause of the outbreak. Support for the fees of further diagnostic work may help identify newly introduced or emerging swine diseases, addressing the risk of missing a significant issue if a definitive diagnosis is not pursued diligently.

The Swine Health Information Center (SHIC) continues to prioritize high impact, urgent return on investment projects to monitor, predict, prepare, and respond to emerging diseases. You are invited and encouraged to provide input into the SHIC 2020 Plan of Work, providing direction for upcoming priorities.
As you consider priorities for emerging disease projects, please use the following questions to inspire your input to SHIC. However, do not limit your consideration to these topics. SHIC desires stakeholders’ most visionary suggestions to benefit the health of the US swine herd.
Please provide your input for the SHIC 2020 Plan of Work to Executive Director Dr. Paul Sundberg. Email [email protected] or call 515-451-6652. Alternatively, you are welcome to share your suggestions with any member of the SHIC Board of Directors.
Please send your contributions to this process by December 13, 2019.

Following the annual meeting of the US Animal Health Association (USAHA) last month, resolutions will be sent to the USDA Animal and Plant Health Inspection Service – Veterinary Services (APHIS-VS) to convey the importance of pork industry issues from the organization’s membership. The US swine industry was well represented on USAHA committees affecting pork production and offering the resolutions, including Transmissible Diseases of Swine, Animal Emergency Management, Animal Welfare, One Health, and National Animal Health Laboratory Network. African swine fever (ASF) was the main topic addressed by several committees with swine industry experts sharing information and was the focus of the keynote address given by Dr. Juan Lubroth, chief veterinary officer for the United Nations Food and Animal Organization. The Swine Health Information Center (SHIC) contributed by providing background and support of the science behind proposed resolutions. The approved resolutions are intended to protect the health of the US swine herd.
RESOLUTION: Adequate Funding for National Animal Vaccine and Veterinary Countermeasures Bank
USAHA urges USDA and State Animal Health Authorities to support a total of $92 million for the National Animal Vaccine and Veterinary Countermeasures Bank (NAVVCB), with a minimum of $20 million for each of the first four years and $12 million in the fifth year, of the funding established in the 2018 Farm Bill to provide adequate number of doses of foot-and-mouth disease vaccine and surge capacity. This $92 million for NAVVCB is to include a reasonable stockpile of foreign animal disease testing kits/reagents needed for outbreak response.
Additionally, the 2018 Farm Bill prevention funding the National Animal Disease Preparedness and Response Program (NADPRP) should not be used to fund current USDA APHIS activities with the states nor should it inhibit full appropriation of the National Animal Health Laboratory Network (NAHLN) laboratory authorization within USDA, National Institute of Food and Agriculture, Food and Agriculture Defense Initiative and APHIS budgets.
RESOLUTION: African Swine Fever/Classical Swine Fever Surveillance Program and Tissues for Official ASF Testing in National Animal Health Laboratory Network Laboratories
USAHA urges USDA APHIS to validate and approve the items listed below. Collectively, these efforts aim to enhance the cost-effectiveness, sustainability, and breadth of coverage provided by the ASF/Classical Swine Fever (CSF) Surveillance Program.
The USDA APHIS ASF/CSF Surveillance Program at USDA NAHLN laboratories shall:
Foreign animal disease (FAD) diagnostic capabilities and capacities at USDA NAHLN laboratories shall:
RESOLUTION: Valid Sampling Methods and Protocols for Feed and Feed Inputs
USAHA urges the Food and Drug Administration Center for Veterinary Medicine and USDA APHIS-VS to work with the US pork industry to develop valid sampling methods and protocols to detect pathogens in foreign feed and feed inputs that can be applied at the point of embarkation to the US or upon arrival at the port of entry.
RESOLUTION: Efficient Diagnostic Sample Validation and Approval for Foreign Animal Diseases of Swine
USAHA urges the USDA APHIS-VS to work with US pork industry to validate and approve swine oral fluids, swine processing fluids, and meat juice for detection of antigen and antibody for CSF, ASF, and foot-and-mouth disease (FMD).
RESOLUTION: Foreign Animal Disease Prevention
USAHA urges the Department of Homeland Security (DHS), United States Customs and Border Protection (CBP) to 1) on a quarterly basis, provide interdiction metrics to pork industry representatives, 2) work with the FMD Cross-Species Team to develop education designed to increase awareness for passengers that are in transit from foreign ports into the US on the importance of protecting agriculture and being truthful on the US Customs Declaration form, 3) work with the FMD Cross-Species Team to develop biosecurity education for travelers diverted for secondary screening after declaring they have been on a farm or in contact with animals in a foreign animal disease positive nation, and 4) modify the US Customs Declaration form to include language regarding a traveler’s proximity to packing and processing plants, live and/or wet markets, research facilities, laboratories, or any other location where there is a likelihood that cross-contamination could occur directly or indirectly between the traveler and animals, fresh animal products, or animal excretions.
RESOLUTION: Evaluating and Recognizing Compartments
USAHA urges USDA APHIS-VS to host a meeting with the US pork industry and State Animal Health Officials to discuss the proposed criteria that will be used to evaluate and recognize livestock/livestock products compartments domestically and internationally.
RESOLUTION: Stop Movement – Criteria for Implementing and Releasing
USAHA urges USDA APHIS-VS to work with the United States pork industry and state animal health officials to develop criteria for implementing and releasing national movement standstills due to the occurrence of a trade and commerce limiting foreign animal disease of swine.

In the December report, the percentage of positive porcine reproductive and respiratory syndrome virus (PRRSV) cases in November was 26.15%, up from 22.65% in October, with increased detection in all age categories. The increase follows the predicted expectation for the period. Porcine epidemic diarrhea virus (PEDV) overall positive cases in November were at 13.97%, up from 10.82% in October. The increased detection of PEDV RNA was above expected, mostly driven by wean-to-market animals. The overall porcine deltacorona virus (PDCoV) percentage of positive cases in November was at 4.18%, up from 2.13% in October. There was a signal for increased detection of M. hyopneumoniae above expected in November. Details on these and other monitoring results are included in the full report.

A new outbreak of African swine fever (ASF) outbreak was reported in wild boar in the Polish province of Lubusz in November. ASF continues to be diagnosed in South Korea where the 34th wild board was reported late in the month. A European Union risk assessment reports a “very high risk” of ASF spreading to nine countries in south-eastern Europe, each currently ASF-free. A foot-and-mouth disease diagnosis in South Africa has suspended that country’s FMD-free zone. Read all the details in the full report.
Copyright 2024 | Swinehealth.org | Website by Heartland Marketing Group