
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments.

Check out the latest episode of SHIC Talk to hear SHIC Associate Director Dr. Lisa Becton discuss the recently completed Japanese encephalitis virus economic assessment with principal investigator Dr. Liz Wagstrom, along with host Barb Determan.

For the first time in the US, highly pathogenic avian influenza has been identified in domestic livestock including goats and dairy cattle. To understand the threat HPAI poses to domestic livestock species, and to inform producers on actions that can be taken to reduce the risk of infection on-farm, the Swine Health Information Center in collaboration with the American Association of Swine Veterinarians will host a webinar on influenza A viruses on Friday, April 19, 2024, from 11:00 am to 12:30 pm CST. To register, click here.
The recent detection and confirmation of HPAI in domestic livestock by USDA has raised questions regarding the emerging threat and potential risks for swine herds. During the webinar, presenters will provide the latest information on influenza A virus, including an overview of the pathogen, global and domestic distribution, research outcomes for HPAI experimental infection in swine, experiences, and perspectives of the dairy industry from the current outbreak, and an outbreak investigational tool for identifying and mitigating biosecurity risks.
Presenters confirmed to date for the webinar include:
Dr. Amy Baker, USDA ARS
Dr. Bailey Arruda, USDA ARS
Dr. Jamie Jonker, National Milk Producers Federation
Dr. Derald Holtkamp, Iowa State University
Presenting timely and responsive webinars to inform the swine industry on potential emerging disease threats is part of SHIC’s mission to protect the health of US swine.
Global swine disease monitoring identified Japanese encephalitis virus, a transboundary disease transmitted by Culex mosquitos, as an emerging threat to the US swine industry. The 2022 JEV outbreak in Australia heightened the need to define the risk this virus poses to US pork production, informing prevention, preparedness, and response efforts. In 2022, the Swine Health Information Center funded a study led by Dr. Natalia Cernicchiaro, Kansas State University, in collaboration with researchers at the USDA ARS Foreign Arthropod-borne Animal Diseases Research Unit, to update her group’s 2018 qualitative assessment estimating the risk of JEV emergence and subsequent transmission in the continental US. Incorporating the latest scientific information and elements into the new study, the updated semi-quantitative assessment evaluated the risk of JEV introduction into seven US regions, its subsequent spread, and economic impact. Study results found the overall risk, reflecting the rate of introduction and economic impact of a JEV incursion, was non-negligible for the south, west, midwest, and northeast regions.
Although currently free from JEV, the US is considered a susceptible region for incursion due to multiple factors. Specifically, the availability of competent insect vectors, susceptible maintenance (avian) hosts, large populations of susceptible and amplifying hosts (domestic and feral pigs), extensive travel and trade to and from JEV-affected countries, as well as similar climatic and environmental conditions as endemic countries fuel susceptibility. To reassess the current risk of JEV emergence into the US, the study sought to update the 2018 assessment and add information regarding transmission post-emergence by incorporating the latest scientific information, updating assumptions, adding data sources, including the recent outbreak data from Australia, and considering regional elements contributing to the risk. Find the industry summary of the JEV risk assessment here.
To perform the updated semi-quantitative JEV risk assessment, the 48 contiguous US states were grouped into five regions based on climate, domestic swine production, and presence of feral swine. Due to their geographic isolation, Hawaii and Alaska were identified as their own respective regions. Using the risk framework, introduction of JEV into the seven US regions was evaluated from the current international regions of JEV distribution (i.e., southeast Asia, Australia, western Pacific rim). Many pathways of entry were considered, including infected vector eggs/larvae via imported goods, infected adult vectors via aircraft, ships, or shipping containers, and infected ardeid birds migrating from the current region of distribution.
Information utilized to update model parameters included data from an updated systematic review of the literature on vector and host competence for JEV. The updated review, assessing literature published from 2016-2022, reported a slightly higher number of articles that focused on host competence and susceptibility to JEV compared to vector competence. This shift in focus could be attributed to, in part, the realization of certain host species’ (e.g., pigs, migratory and domestic birds) high susceptibility to JEV. Additional sources of information for the risk assessment models included global databases, scientific literature, and expert opinion obtained from the JEV advisory group and project collaborators. The JEV advisory group consisted of swine producers, veterinarians, entomologists, researchers and stakeholders from the US and Australia with knowledge on commercial swine production, JEV, and the 2022 Australian outbreak.
Summary output parameters calculated by this risk assessment included the rate of introduction, estimated epidemic size, and the overall risk estimate. The highest risk scores for the rate of introduction were deemed moderate and associated with the entry of adult mosquitoes in ships into the US south (AL, AK, DE, Fl, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA) and west (CA, ID, OR, WA) regions, and via mosquito eggs/larvae in imported tires into the northeast (ME, MA, NH, NY, VT) and west regions. The overall risk, which reflects the rate of introduction and economic impact of a JEV incursion into the region at risk, was very high for the south, moderate for the west, and very low for the northeast and the midwest regions.
In summary, based on the results from this risk assessment, the US south region had the highest risk of introduction and impact and should be prioritized for preparedness, in addition, the west and northeast regions should promote surveillance to facilitate early detection. This qualitative risk assessment is crucial to prepare for the threat of an emerging disease and protect the swine industry from suffering production and economic losses. Defining the US regions at greatest risk for JEV is an important step towards US prevention and preparedness as well as allocation of efforts for surveillance.
Japanese encephalitis virus, a transboundary emerging disease risk for US introduction, carries with it potential economic implications greater than $500 million per a recent economic assessment white paper (Cook et al., 2024). Transmitted by the bite of infected mosquitos, JEV can cause severe disease in pigs, horses, and humans. While historically endemic to Asian and western Pacific regions, the 2022 JEV outbreak in Australia raised concerns of a renewed threat to the US pig industry and public health.
For US preparedness, it is critical to establish a reliable way to detect JEV in swine samples prior to an introduction or outbreak. Principal investigator Dr. Rahul Nelli and co-investigator Dr. Phil Gauger, Iowa State University, recently completed a Swine Health Information Center-funded project to establish RT-rtPCR assays for JEV and its five genotypes, comparing the newly established test with a previously published assay in the process. Development of a JEV test for US veterinary diagnostic labs that can accurately and efficiently detect multiple JEV genotypes will help the US pig industry monitor and respond rapidly to a potential JEV incursion.
A potential outbreak of JEV in the US pig population can have detrimental economic and public health impacts. It is crucial to be vigilant and establish a screening method to detect the virus early when clinical signs suggest that JEV could be a differential diagnosis. Recently completed, the full report of the project entitled, “Establish and validate RT-rtPCR for detecting JEV in porcine samples,” can be found here.
Findings from this project suggest the newly developed novel RT-rtPCR assay is more specific than previously published assays and can detect all five JEV genotypes (G-1, -2, -3, -4, -5) accurately and efficiently. The new assay also has a low detection limit, meaning it can detect very small amounts of the virus. Further, the project confirmed that the novel JEV PCR assay does not cross-react with other viruses endemic to the US swine herd, such as PRRSV, PPV1, PPV2, PEDV, TGEV, PDCoV, PoAstV3 or PoAstV4. Validation of genotype-specific RT-rtPCR assays and evaluation of assay performance on known status clinical samples in collaboration with Australian swine industry stakeholders is ongoing.
Before the assay developed in this project can be used for routine diagnostics, it must be further validated for diagnostic specificity and sensitivity utilizing the American Association of Veterinary Laboratory Diagnosticians and National Animal Health Laboratory guidelines. To accomplish this, a collaborative effort will be undertaken with known status JEV samples from the Australian outbreak. This collaborative research aims to ensure that the assay is accurate and reliable in detecting JEV in various conditions and across different clinical samples. By validating the assay using these methods, greater confidence is achieved in its ability to help identify and prevent the spread of JEV in pigs. Early and accurate diagnosis of JEV is crucial for rapid response efforts to reduce the potential disease impact on humans and the production impacts for pork producers.

In the eighth annual report to the Swine Health Information Center, which provides the primary funding for its work, Morrison Swine Health Monitoring Project leader Dr. Cesar Corzo of the University of Minnesota detailed project outcomes for its three primary objectives. MSHMP monitors trends in pathogens incidence and prevalence, conducts prospective monitoring of PRRSV sequence evolution and impact, and expands participation of producers to increase relevance and deliverables to the swine industry.
Swine pathogens PRRSV, PEDV, PDCoV, SVA, and central nervous system associated viruses are routinely monitored and compared to historical trends. Additional investigations were undertaken to evaluate pathogen patterns, epidemiology and herd stabilization post-infection. MSHMP investigated the relationship of the PRRS RT-PCR positivity rate between breeding and growing pigs during 2015 to 2020 from MSHMP participants. A correlation was identified in the time lapse between positive submissions from nursery/finisher samples and adult/farrowing samples. The correlation found that adult/farrowing positivity follows the same trend as the nursery/finisher positivity with a one-week lag. However, the data was interpreted with caution as no account for spatial distribution or PRRS virus sequence information was included. Consequently, MSHMP was unable to conclude that the increase in PRRS positive rate in breeding herds is due to an increase in positivity rate in growing pigs.
In another investigation, MSHMP assessed the frequency of PRRS outbreaks in breeding herds managing mortality either through composting, incineration, or rendering. The goal of the investigation was to identify associations between carcass disposal methods and PRRS occurrence. Results of the study include analysis of 133 breeding sites from eight production systems to date. One finding included a higher numerical rate of ≥1 PRRS outbreaks on sites using compost (29.7%) compared to sites using rendering (21.1%). However, when accounting for variables such as production system, average inventory, air filtration status, and state, composting was not associated with a higher number of PRRS outbreaks when compared to rendering (p = 0.67). Further assessment of data is warranted once additional data is received from participating systems and the study is evaluated over an extended time frame.
Regarding PEDV, MSHMP quantified the time that positive herds required for stabilization across both the epidemic phase (May 1, 2013 – December 31, 2014) and the endemic phase (January 1, 2015 – June 30, 2023). Results concluded that those infected during the epidemic phase of the disease in the US took nine weeks longer to reach stability than those infected during the endemic phase, reflecting industry progress in PEDV control. Being able to estimate time to stability can assist producers and their veterinarians develop an effective herd plan for PEDV management and potential elimination.
For PRRSV outbreaks, MSHMP provided an extensive dataset to report the spatial-temporal distribution of strains similar to the outbreak strain, providing invaluable information during outbreak investigations. MSHMP characterized the epidemiological situation of an emerging PRRS virus (L1C-124) that was thought to be virulent and fast spreading. A total of 382 case sequences were identified across 16 production systems. Most sequences originated from breeding sites (n = 223) compared to grow/finish sites (n = 127), with the sequences originating from 118 unique sites (73 grow/finish, 37 breeding, and eight unknown). Even though the virus did disseminate, it did not reach the transmission speed and magnitude of spread for other recently identified PRRS variants. Clinical impact was also characterized and found to be similar to the recent L1C-144. Continuous monitoring and assessment of emerging PRRS strains is critical to manage transmission and highlights the need for focus on biosecurity practices to prevent spread.
During 2022-2023, MSHMP added four production systems to their ongoing collection of swine health data. The project’s database now has 1,274 sow farms housing approximately 3.8 million sows, 3,318 growing pig farms with 12.2 million pig spaces, and 48 boar studs totaling 12,799 boars. The addition of new groups and voluntary participants allows for improved monitoring to assess the role grow finish populations play for area/regional disease pressure.
MSHMP’s first publicly available website officially launched in Q3 of 2023 and has been continuously updated. This website is designed to optimize MSHMP output dissemination within both the participant and industry communities. The website has been designed with 10 comprehensive sections, including Home, About, History, People, Reports, Outreach, Ongoing Projects, News, Resources, and Contact Us. The website has been accessed by individuals from 41 countries with the highest number of users coming from the US, China, and Spain.
PRRSV and PEDV cumulative incidence data graphs from MSHMP are also now included in SHIC’s monthly enewsletter along with a link to sign up to participate in the program and/or receive weekly reports.

Since December 2017, more than 80 Global Swine Disease Monitoring Reports have been developed by a team at the University of Minnesota, now led by Dr. Maria Sol Perez Aguirreburualde. Funded by the Swine Health Information Center as part of its mission to identify emerging disease threats, the monthly reports are published in the SHIC newsletter and serve as a frequently accessed resource for the swine industry on the SHIC website. Reports are built with near real-time global surveillance of swine diseases for their content and rely on a network of global collaborators to expand and verify regional information. With renewal, the GSDMR will continue and expand in 2024 with a new online dashboard to display the global distribution of priority swine diseases in near real-time.
The GSDMR uses a continually updated procedure of screening to identify and score swine disease related events that may represent a risk for the US swine industry and reports those results on a monthly basis. Both official and unofficial information sources from primary or secondary platforms are collected, reviewed, then organized by disease and geographical region. A technical team subsequently synthesizes and frames each disease section, facilitating its interpretation by the audience reviewing the GSDMR.
After a multi-step review phase in which data and information is verified, edited, and/or expanded, in collaboration with key technical informants and a network of US and international stakeholders, a report describing the surveillance outcome is made available to SHIC. In extraordinary circumstances in which a rapid response is indicated, an immediate release of health event data is developed for rapid sharing with the pork industry (e.g., the first African swine fever outbreak in China, first ASF outbreak in Belgium, first classical swine fever outbreak in Japan in 25 years).
From November 2022 through April 2024, 17 GSDMRs were produced, highlighting the continuous expansion of ASF in Asia, Europe, and Latin America. Currently, the three USDA-classified tier 1 reportable foreign animal diseases of swine (ASF, CSF, and foot-and-mouth disease) are included in the report. Additional swine diseases listed in the SHIC disease matrices, such as influenza and pseudorabies, are also included when appropriate after considering the epidemiological context of the event.
In 2023, a new section was incorporated into the GSDMR to provide specific regional disease information for swine industry stakeholders. In this feature, entitled “Focus on,” comprehensive snapshots of current knowledge of swine infectious diseases throughout regions were delivered quarterly. This section zoomed in on countries with emergent or consolidated pig production sectors. Feature goals were to build a temporal and spatial context regarding the dynamic changes of swine infectious diseases to support the understanding of point-in-time changes captured in the monthly report. Additionally, this new feature creates awareness of the risk dynamic to the US swine herd. A credible and consistent source of robust information is a crucial element for disease prioritization and for the development of risk-based preventive strategies to protect the swine industry.
The GSDMR is consistently one of the most accessed items in the monthly SHIC newsletter. In 2023, there were 7046 GSDMR page views on the SHIC website, making it one of the most visited on www.swinehealth.org.
The US swine industry is vulnerable to the introduction of emerging pathogens and routine swine disease monitoring provides early warnings of global disease challenges that could negatively impact US swine producers. GSDMR staff strive to continuously improve the model through developing private-public-academic partnerships to support near real time identification of hazards. This contributes to the mission of identifying disease risks and adds practical insight into the tools used by different stakeholders for disease prevention and control. Ultimately, the goals of the GSDMR are to continue contributing to identification of risks and raising awareness to support decision making process around current prevention and mitigation strategies for emerging disease introduction in the US.

A project funded by the Swine Health Information Center focusing on the role of vehicle movement in swine disease dissemination has recently been published in the Journal of Preventive Veterinary Medicine. Principal investigators Drs. Gustavo Machado and Jason Galvis, North Carolina State University, studied a novel method to analyze the role of vehicle movement in swine disease spread by accounting for the variability around pathogen stability and vehicle cleaning effectiveness. Specifically, the study investigated the moving vehicle’s role in disease spread from farm to farm by considering 1) the factors that may affect the pathogen stability on the contaminated vehicle and 2) the efficacy of the procedure to clean and disinfect the contaminated vehicle.
Knowing there are many methods for swine disease spread, the objective of this study was to increase understanding around the risk of vehicles traveling to and from swine farms for disease transmission across the US pork industry. A goal was to define the contribution of moving vehicles in disseminating disease from farm to farm. The investigators collected vehicle movement data and vehicle cleaning efficacy data for the project. Using the African swine fever virus as the pathogen of interest in the model, they reconstructed a vehicle movement network to evaluate the possible dissemination of the ASFV between farms.
Investigators collected a year’s GPS data on 823 vehicles moving between 6363 production sites across two US regions. Region one consisted of 1974 commercial swine farms managed by six production companies, and region two consisted of 4389 commercial swine farms managed by 13 production companies. Farms were classified into 24 types based on swine production phase and simplified into categories such as a sow farm, which contained breeding age animals. Five vehicle types were named by the type of use to include feed vehicle, pig-farm vehicle, pig-market vehicle, crew vehicle, and undefined vehicle. For each vehicle, 12 months of daily GPS tracker records were collected. Additional information was also collected, including company-owned cleaning stations for transportation biosecurity analysis and incorporation of vehicle cleaning and disinfection.
This study extended and updated a previously developed method for evaluating disease transmission across vehicle contact networks. For the current study, Drs. Galvis and Machado considered both vehicle cleaning and disinfection efficacy uncertainty along with ASFV stability decay in the environment.
“Our study revealed that although efficient cleaning and disinfection measures affected the number of farms connected through vehicle movements, simulations with 100% cleaning and disinfection still resulted in 88% of farms being in contact over one year,” investigators wrote. “Importantly, achieving 100% cleaning effectiveness reduced the risk of between-farm contacts only when the ASFV stability was low (≤0.2). Conversely, there was an insignificant reduction in the number of between-farm contacts when the ASFV stability was still high (>0.8).” ASFV stability was measured on a scale from 0 to 1, with 1 being the greatest stability.
The investigators discovered vehicles visited different swine production companies’ sites, enhancing the potential for between-company dissemination in the new study as well. In their report, the investigators found that in the absence of cleaning, vehicles connected up to 2157 farms in region one and 437 farms in region two. In region one alone, vehicles transporting feed connected 2151 farms, vehicles transporting pigs to farms connected 2089 farms, vehicles transporting pigs to market connected 1507 farms, undefined vehicles connected 1760 farms, and vehicles transporting personnel connected three farms.
Simulation results of the model provided valuable insight into how contact networks between farms could be reduced through vehicle cleaning, which was assumed to be 100% effective. Specifically, contact networks were reduced as follows:
“The results of this study showed that even when vehicle cleaning and disinfection are 100% effective, vehicles are still connected to numerous farms. This emphasizes the importance of better understanding transmission risks posed by vehicles to the swine industry and regulatory agencies,” they stated.
This study enhances understanding of the role of transportation vehicles in spreading diseases between farms and the risks involved. The new methodology introduced in this study can be used to develop novel disease control strategies, including rerouting vehicles based on their infection and risk status.

Dr. Paul Sundberg, who served as executive director of the Swine Health Information Center from 2015 – 2023, was honored at the recent Pork Industry Forum with the Distinguished Service Award. This well-deserved recognition celebrated his many contributions to the US pork industry, including work at the National Pork Board as well as taking on the responsibility for the Swine Health Information Center in 2015.
Read more about the Distinguished Service Award and Dr. Sundberg’s career here.
The Swine Health Information Center echoes the accolades shared in the award presentation and offers its sincere thanks to Dr. Sundberg for his contributions to the industry he served.

This month’s Domestic Swine Disease Monitoring Report brings the new influenza A virus state-level monitoring dashboard. The newly implemented tool aids veterinarians and producers in identifying if the percentage of influenza A positive cases is above expected in their region on a monthly basis. Also, the report brings information about the wean-to-market positivity remaining high for PRRSV, with some regions such as Iowa and Indiana having a percentage of positive submissions above the expected. For enteric coronaviruses, PEDV and PDCoV positivity decreased during March, mainly in the wean-to-market category. However, in the state-level monitoring, the overall positivity is above the expected for PEDV in Kansas and PDCoV in Minnesota and Missouri. Influenza A virus substantially increased positivity for the wean-to-market farm category, with 51% of the IAV-positive cases being lung submissions. In the podcast, SDRS hosts talk with Dr. Peter Schneider (Innovative Agriculture Solutions) about managing animal health in highly swine dense areas.

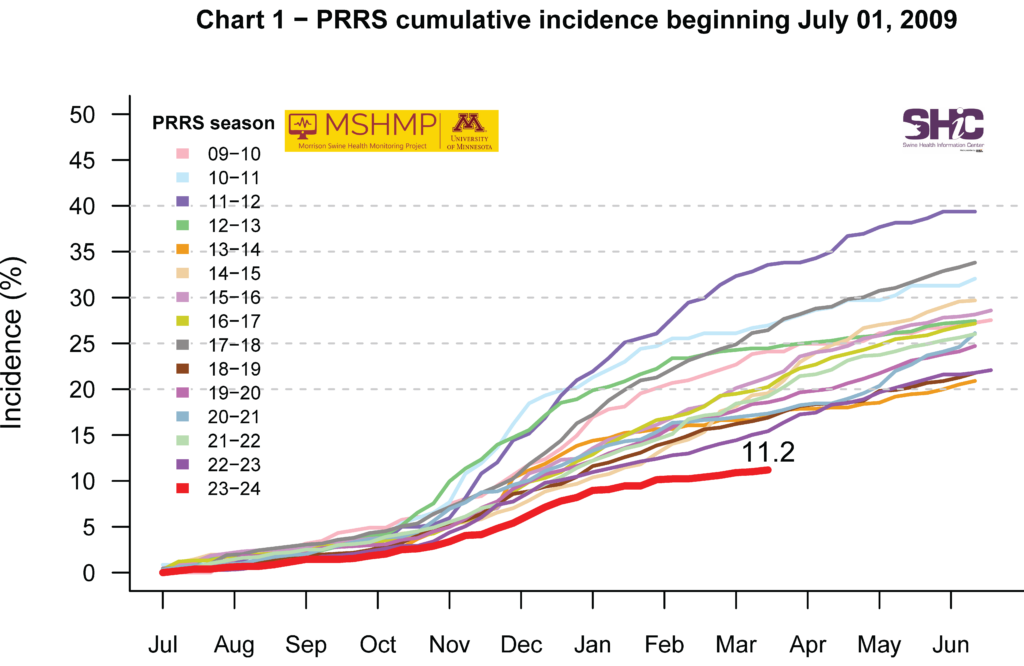
PRRS Cumulative Incidence for MSHMP Beginning July 1, 2009
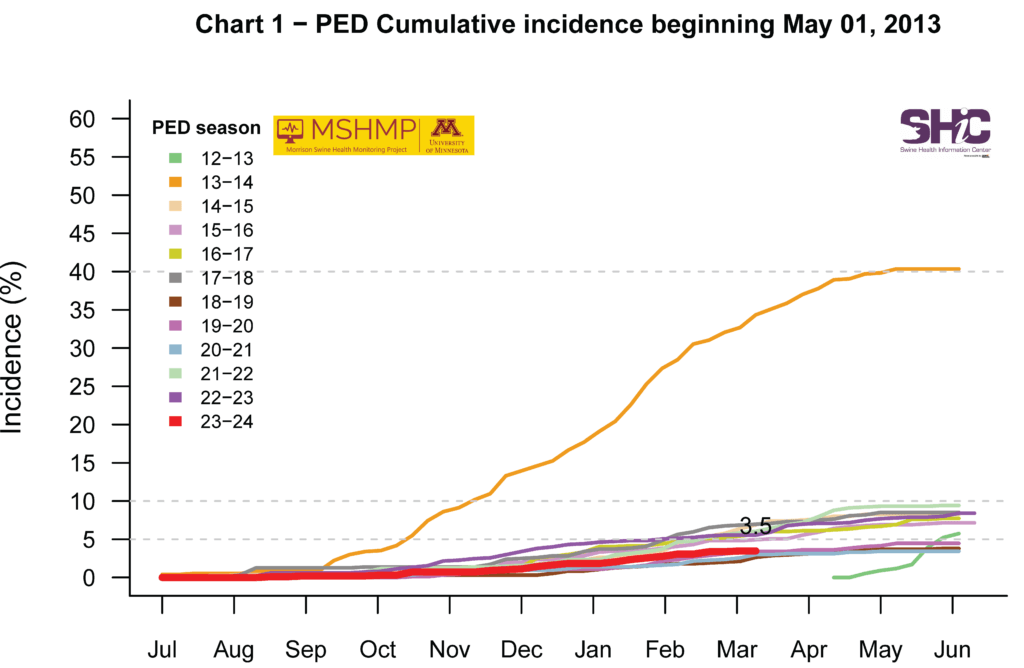
PEDV Cumulative Incidence for MSHMP Beginning May 1, 2013
Copyright 2024 | Swinehealth.org | Website by Heartland Marketing Group