
 Swine Disease Global Surveillance Report
Swine Disease Global Surveillance ReportThursday, August 20, 2018
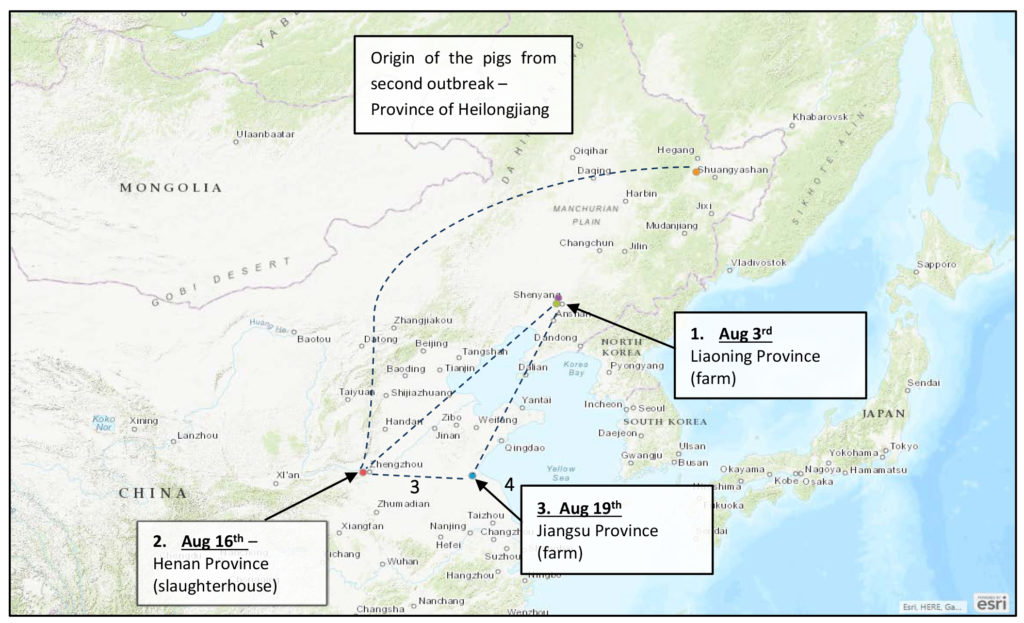
A third case of ASF has been reported in a farm in the in Haizhou District, part of the city of Lianyungang, in the Jiangsu Province (map). This area is approximately 350 miles east of the 2nd case, reported last Thursday at a slaughter plant in the city of Zhengzhou, Henan province.
Starting on August 15, 2018, pigs started dying from an unknown etiology on one farm in Haizhou District, Lianyungang city, Jiangsu province, China. In this case there have been 615 pigs reported as infected with 88 pigs having died from confirmed ASF. After the outbreak, a supervision work group was promptly dispatched to the Jiangsu Province by the Ministry of Agriculture and Rural Affairs. Strict blockade, disinfection, killing and disposal of the animals and relevant contaminated materials, and movement control measures were conducted in the farm. The local government launched the African swine fever contingency plan and emergency response according to the Standards & Protocols for ASF. All live pigs and animal products were prohibited to enter and exit the area.
The three cases now reported in three provinces, and a 4th northern province related to the second event, providing pigs that were infected by delivery to the plant in Zhengzhou.
These reported incidents have occurred over a large swath of China, containing tens of millions of pigs.
From China’s current outbreak report to the OIE, dated August 19:
“After the African swine fever (ASF) outbreak, a supervision work group was promptly dispatched to the Jiangsu Province by the Ministry of Agriculture and Rural Affairs. Strict blockade, disinfection, killing and disposal of the animals and relevant contaminated materials, and movement control measures were conducted in the farm. The local government launched the African swine fever contingency plan and emergency response according to the Standards & Protocols for ASF. All live pigs and animal products
were prohibited to enter and exit the area.”
Pigs in China are reared across a wide range of farms, including units that are similar to modern US production systems. However, much of the pork consumed is still from small household units that are fed the food scraps of a family or neighborhood. Likewise, the slaughter and sale of pigs is often through modern slaughter facilities and supermarkets, but much of the pork is sold without refrigeration, with local slaughter and sales from neighborhood vendors.
The swine industry has never seen an ASF outbreak in such a production landscape, and control measures are untested.
The Chinese industry has had difficulties in controlling FMD and CSF, and has relied heavily on the use of vaccines. As a vaccine is not available for ASF, the industry is thus reliant on heightened biosecurity, rapid diagnosis, complete isolation, and then elimination of infected pigs and contaminated materials.
Copyright 2025 | Swinehealth.org | Website by Heartland Marketing Group